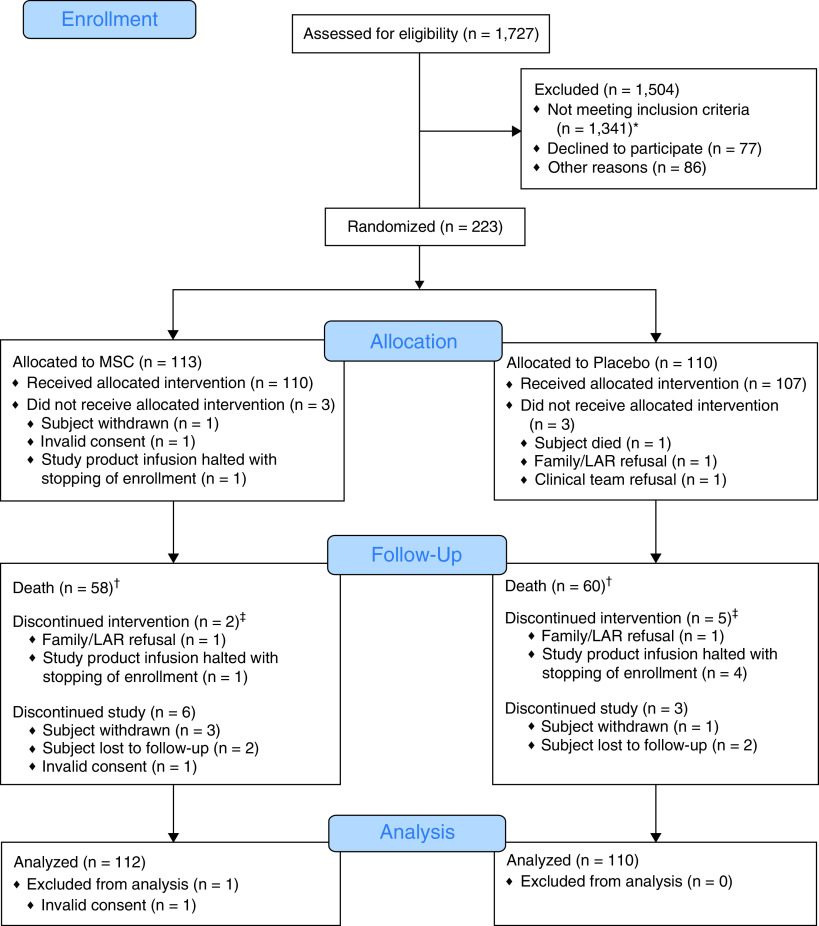
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram. *The most common reasons for not meeting inclusion/exclusion criteria included not having moderate and/or severe acute respiratory distress syndrome or requiring mechanical ventilator support (n = 533) and intubation greater than 72 hours (n = 208). †Three subjects discontinued intervention because of death (MSC = 1 and placebo = 2). ‡Discontinued intervention means the second infusion was not administered. LAR = legally authorized representative; MSC = mesenchymal stromal cell.