Abstract
Ethiopia is home to one of the richest and most unique assemblages of fauna and flora on the African continent. Contained within its borders are two major centers of endemism, the mesic Roof of Africa (also known as the Ethiopian Highlands) and the arid Horn of Africa, resulting from the country’s varied topography and consequent geographic isolation. These centers of endemism are crucial to global conservation as evidenced by their classification within the Eastern Afromontane and Horn of Africa biodiversity hotspots, respectively. Ethiopia’s diverse ecosystems and the biodiversity they contain are increasingly threatened by climate change and the growing impacts of Africa’s second largest human and largest livestock populations. In this paper, we focus on several key areas of recent and ongoing research on Ethiopian biodiversity that have broadened our understanding of nature and its conservation in Africa. Topics explored include the behavioral ecology of Ethiopia’s large social mammals, the ecology and conservation of its unique coffee forests, and Ethiopian approaches to community conservation, fortress conservation, and nature-based solutions. We also highlight the increasing prominence of Ethiopian scientists in studies of the country’s biodiversity in recent decades. We suggest promising avenues for future research in evolutionary biology, ecology, systematics, and conservation in Ethiopia and discuss how recent and ongoing work in Ethiopia is helping us better understand and conserve nature in the human-dominated landscapes of Africa and other tropical regions today.
Keywords: biodiversity hotspots, climate change, community conservation
Sub-Saharan Africa possesses a spectacularly diverse array of fauna and flora while also boasting a burgeoning human population that must find ways to effectively manage the finite natural resources upon which all life on the continent depends (1). Part of the answer lies in successfully addressing the question—how can we best study and conserve nature in the human-dominated landscapes of sub-Saharan Africa today? The ancient nation of Ethiopia in northeastern Africa offers some unexpected insights. Ethiopia is today more widely known for its remarkable past cultural achievements (2, 3) and fossils of extinct human relatives (4 –6) than for its extraordinary living biological riches. Situated at the crossroads between two major centers of endemism and biodiversity hotspots—the mesic Roof of Africa or Ethiopian Highlands (Eastern Afromontane hotspot) and the arid zone of the Horn of Africa (Horn of Africa hotspot)—Ethiopia possesses some of Africa’s most unique yet least known biodiversity (7 –9).
The unusual geology and rugged topography of the Ethiopian Highlands have isolated much of the country from the rest of Africa, resulting in the evolution of many endemic plants and animals (10). Despite having one of the oldest written languages in Africa and a meticulously recorded history (11), until the 1970s, little was documented about Ethiopia’s faunal and floral diversity (12). Given the high degree of species richness and endemism and the wide array of habitat islands found within its borders, Ethiopia is a fruitful testing ground for many questions in evolutionary biology, behavioral ecology, and conservation biology. There is also a particular urgency to biological research in Ethiopia because many of its natural areas are threatened by its large, rapidly increasing human and livestock populations and by climate change (7, 13). To encourage and provide context for more research on Ethiopia’s globally unique biological resources, here, we discuss several key themes of recent and current research, assess the contributions of this work to our understanding of science and conservation in Africa, and explore promising future directions for research in Ethiopia.
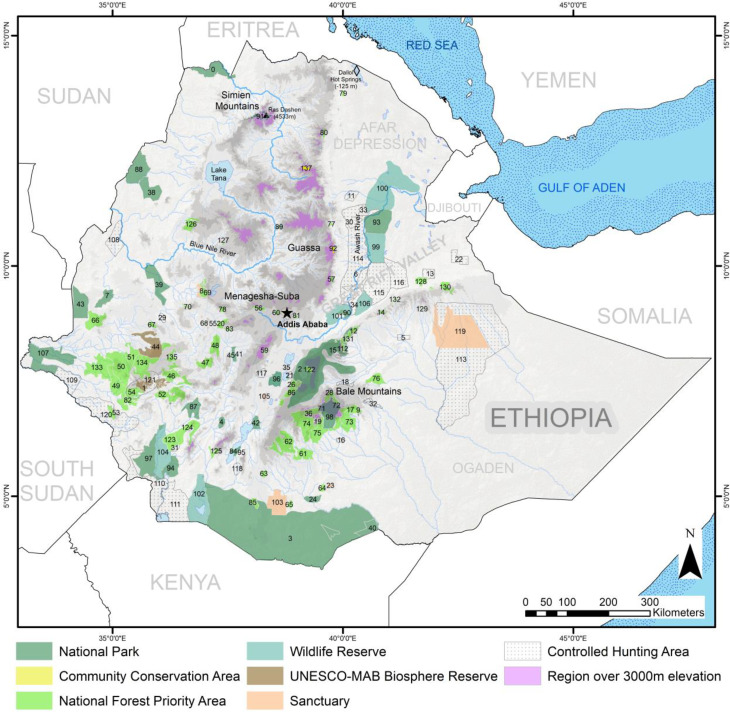
Ethiopia’s impressive biological diversity is due in large part to a fortuitous mix of geography, topography, and geology. The country lies entirely within the tropics and exhibits unusually wide variation in altitude and climate, ranging from one of Africa’s lowest and hottest locations (Dallol hot springs in Danakil Depression, −125 m below sea level) to one of its highest and coldest points (Ras Dashen in the Simien Mountains National Park (SMNP), 4,533 m above sea level) (Fig. 1) (14). Ethiopia also contains the source of the Blue Nile, one of the two major tributaries of Africa’s longest river, and possesses the largest contiguous area of high-altitude land in Africa—the Ethiopian Highlands—accounting for 80% of all land above 3,000 m on the continent (15) (Fig. 1). Beginning as a single mountain massif that later split into two by tectonic activity and the development of the Great Rift Valley during the Miocene (14), today, both halves (northern and southern) of the Ethiopian Highlands support many endemics owing to their geographical isolation and the unique climate of the region (10). In addition to its extensive Highlands, Ethiopia possesses large expanses of lower-elevation ecosystems, including forests, swamplands, and the deserts and semidesert grasslands and scrublands of the Horn of Africa, which also harbor their own unique (and less studied) constellation of endemic plants and animals (8, 16).
Fig. 1.
Map of Ethiopia’s protected areas which cover 14% of the country’s landmass and contain much of its biodiversity. Numbers correspond to details about each protected area in the key provided in SI Appendix, Table S1.
Although inventories of its biodiversity are far from complete for some taxonomic groups, Ethiopia has been provisionally ranked as the 12th most biodiverse country in Africa (17). Within its borders are known to exist ~6,000 species of plants (600 endemic) (18 –20), 863 species of birds (19 endemic) (21), 271 species of mammals (45 endemic) (22, 23), 174 species of fish (42 endemic) (24), 64 species of amphibians (26 endemic) (25), 242 species of reptiles (4 endemic) (22), and 1,225 species of arthropods (7 endemic) (26) ( SI Appendix, Fig. S1). Much of this biodiversity is concentrated in the Ethiopian Highlands, in the high-elevation heart of the country, which stretch north into Eritrea. Flagship endemics of the Highlands include the Ethiopian wolf (Mammalia: Canidae: Canis simensis), gelada monkey (Mammalia: Cercopithecidae: Theropithecus gelada), Bale monkey (Mammalia: Cercopithecidae: Chlorocebus djamdjamensis), Walia ibex (Mammalia: Bovidae: Capra walie), blue-winged goose (Aves: Anatidae: Cyanochen cyanoptera), giant lobelia (Eudicots: Campanulaceae: Lobelia rhynchopetalum), and torch lily (Eudicots: Asphodelaceae: Kniphofia foliosa) ( SI Appendix, Fig. S2). A second less species–rich center of endemism, the arid zone of the Horn of Africa, is situated in the lowlands of eastern Ethiopia and spreads south into Kenya and east into Somalia (8). Flagship endemics of the Horn of Africa that occur in Ethiopia include the desert warthog (Mammalia: Suidae: Phacochoerus aethiopicus), naked mole-rat (Mammalia: Heterocephalidae: Heterocephalus glaber), dibatag (Mammalia: Bovidae: Ammodorcas clarkei), Degodi lark (Aves: Alaudidae: Mirafra degodiensis), and scented frankincense (Eudicots: Burseraceae: Boswellia ogadensis) and myrrh (Eudicots: Burseraceae: Commiphora guidottii).
Ethiopia’s biodiversity is today threatened by rapid human population growth and land use change (7, 8). Ethiopia has Africa’s second largest human population despite ranking only 10th in total land area. Over the past 70 y, Ethiopia’s population grew from ~18 million to ~123 million and is currently increasing by 2.4% per year (27). Ethiopians possess Africa’s largest livestock population, and pressures for more grazing and farmland have led to enormous changes in land cover, especially in the Highlands, home to 85% of Ethiopia’s people and 75% of its livestock (13). Between 1990 and 2020, Ethiopia’s naturally regenerating forest cover declined by 16% (28). Over the past decade, its annual percentage of forest wood removal (3%) was the highest in Africa (28). Most of this forest clearance was carried out to facilitate agricultural uses of the land (29), and soil degradation and drought have become increasingly widespread problems (30). Areas of high biodiversity occur on both government-owned and collectively owned community lands, creating a complex matrix for conservation planning and oversight. Multiple efforts at land reform have also been undertaken over the past half century following regime changes, but dissatisfaction with land policies and ownership issues remains (31), also complicating the conservation landscape.
Thus, with its large and growing human and livestock populations, widespread poverty, civil strife, complex land policy issues, and mounting pressure to exploit its natural resources for economic development, there are few countries where the conservation of biodiversity poses a greater challenge than Ethiopia. Fortunately, an ongoing major effort to train Ethiopian scientists at the PhD level has been successful both within Ethiopia’s rapidly growing university systems and abroad (32). This critical juncture in Ethiopia’s economic and scientific development—marked both by a civil war and the COVID-19 global pandemic—represents a vital time to reflect on what has been done to study and conserve the country’s biodiversity to both draw lessons from past experience and suggest directions for future research. Below, we summarize important recent and ongoing developments and provide suggestions for fruitful avenues of research. In particular, we highlight three areas of intensive research in Ethiopia that have contributed unique insights to our understanding of the biodiversity of Africa and its conservation. We also highlight the increasing prominence of Ethiopian scientists in studies of the country’s biodiversity in recent decades. Our review is not meant to be exhaustive or encyclopedic but seeks to highlight those areas in which research in Ethiopia has made unique or important contributions to our growing knowledge of nature in the human-dominated landscapes of sub-Saharan Africa and to encourage new researchers (from Ethiopia and abroad) to consider focusing on the many unexplored avenues for potential study in Ethiopia.
Behavioral Ecology of Ethiopia’s Large Social Mammals.
Since intensive research began on Ethiopia’s fauna in the 1960s, the behavior and ecology of its large social mammals has been a subject of particular interest (33 –35). The nonhuman primates have been especially well represented in past and present research efforts, fitting given that so many fossils of our earliest hominin ancestors have been discovered in Ethiopia. Interest in Ethiopian primates stems not only from their taxonomic uniqueness (they include an endemic genus, two endemic species, and many endemic subspecies (36)), but also the adaptation of several taxa to unusual habitats for primates, including sweltering deserts (37), frigid alpine grasslands (38), and cold montane bamboo forests (39). Understanding how nonhuman primates have adapted to these extreme environments can provide important insights into human adaptations to these habitats as well (40). Here, we briefly review notable research on Ethiopia’s primates and on another well-studied large social mammal, the Ethiopian wolf, endemic to Ethiopia’s alpine grasslands (41). We also highlight new research on Ethiopia’s endemic ungulates and the recently identified cryptic species, the African wolf (Mammalia: Canidae: Canis lupaster).
Long-term studies of individually known animals over their life span are critically important to understanding the ecology and evolutionary biology of long-lived species, yet keeping these projects running year after year without interruption is extremely difficult (42). In early 2020, there were, notably, three ongoing, long-term (>10 y), individual-based studies of wild primates in Ethiopia—two on geladas in the Highlands (43, 44) and one on hamadryas baboons in the desert (45). All three have been suspended or scaled back amid uncertainty related to the COVID-19 pandemic and civil war, reflecting logistical limitations inherent to long-term research projects during times of instability. These three projects share a common focus on longitudinal monitoring of individually known animals to obtain insights into the extent and causes of variation in fitness across individuals and populations adapted to life in unusually harsh environments. They also share a focus on primates that form uncommonly complex, multitiered societies consisting of many (often a dozen or more) polygynous reproductive units that move around together or share a common food supply. Studies of such peculiar aggregations of animals can shed light on the evolution of other multilevel societies, including those formed by humans, elephants, and many cetaceans (46, 47).
One interesting theme to emerge from these studies is the diverse strategies (or counterstrategies) that each sex employs to pursue its own reproductive agenda, sometimes at the expense of the other sex. Soon after assuming reproductive control of a harem of females from another male, male geladas and hamadryas baboons not only commit infanticide (48, 49), but also feticide by inducing spontaneous abortions among most of the pregnant females (44, 45). Both tactics force females to terminate investment in offspring sired by other males. Females, in turn, may adopt a variety of counterstrategies, including producing sexual swellings and exhibiting estrus behaviors during lactation (50). The value of longitudinal studies like these is that they may uncover behavioral adaptations not seen in shorter-term studies, including those spanning the reproductive careers of individual males and females across the entire life span.
Disease ecology is another area in which long-term studies of Ethiopia’s large social mammals have made important contributions. Evidence of fitness impacts of tapeworm-associated disease has recently been documented in gelada populations at both the Guassa Community Conservation Area and SMNP (Fig. 1). Specifically, geladas with coenuri (cysts or swellings) caused by Taenia serialis (Cestoda: Taeniidae) experienced elevated mortality and reduced reproductive rates relative to individuals without coenuri (43, 51). Moreover, Ethiopia’s longest ongoing wildlife study—on endangered Ethiopian wolves in the Bale Mountains National Park (BMNP) (Fig. 1)—has documented the central role infectious disease, particularly rabies and canine distemper, plays in regulating the species’ population dynamics (52, 53). During >30 y of monitoring, at least four outbreaks of these viruses have occurred, each resulting in population declines of 43 to 75% (53, 54). The extinction threat these outbreaks pose to Ethiopian wolves has led to the development of a remarkable integrated disease management strategy involving rabies vaccinations of hundreds of wolves and tens of thousands of domestic dogs (the likely reservoirs for the virus) in surrounding areas (55). This disease management strategy offers a pioneering model for applied conservation of other endangered canids globally.
Another exciting development during the past decade is that Ethiopia’s long-neglected forest-dwelling primates are finally being studied. These studies, led by Ethiopian scientists, have provided the first detailed insights into the lives of several of Ethiopia’s endemic primates, focusing especially on the impacts of habitat fragmentation and disturbance on their behavioral ecology. Recent research on typically bamboo-eating Bale monkeys (56, 57), folivorous Omo River black-and-white colobus monkeys (Mammalia: Cercopithecidae: Colobus guereza guereza) (58), and frugivorous Boutourlini’s blue monkeys (Mammalia: Cercopithecidae: Cercopithecus mitis boutourlini) (59) suggests these Ethiopian endemic forest primates can cope with some degree of habitat degradation by altering their diets and activity patterns. This flexibility is encouraging given the various anthropogenic threats Ethiopia’s forest primates face, but only continued study of these populations can reveal their long-term potential to cope with the continuing degradation of their habitats.
Interspecific competition involving Ethiopia’s large mammals is another ongoing focus of Ethiopian scientists. Recent work suggests that three of Ethiopia’s endangered endemic ungulates, Walia ibex (60), mountain nyala (Mammalia: Bovidae: Tragelaphus buxtoni) (61), and Swayne’s hartebeest (Mammalia: Bovidae: Alcelaphus buselaphus swaynei) (62), experience feeding competition from domestic livestock. All three species have small remaining populations and limited dispersal opportunities (62 –65), and continued increases in sympatric livestock populations may put them at even greater risk through both resource competition and disease transmission (62). Furthermore, research on the newly described cryptic African wolf (66) suggests that this relatively abundant species exhibits niche overlap and dietary competition with endangered Ethiopian wolves (67, 68).
Ecology and Conservation of Coffee Forests.
Among African nations, Ethiopia’s plants and vegetation types are unusually well described. Approximately 6,000 species have been identified across 12 vegetation types, and exhaustive profiles can be found in the Flora of Ethiopia and Eritrea (18) and the Atlas of the Potential Vegetation of Ethiopia (9). The ecology and conservation of these plants and vegetation types have been studied to varying extents, with forests and woodlands receiving the most research attention, while the desert and semidesert scrublands and the Afroalpine and Ericaceous belts have been relatively understudied (69). Rather than attempting to systematically review this extensive literature, here, we focus on the ecology and conservation of a subcategory of vegetation (within the moist evergreen Afromontane forest type) of great biodiversity and financial importance to Ethiopia and the world—the coffee forests of southwest Ethiopia.
Interest in the ecology and conservation of the world’s coffee forests has increased in recent decades as worldwide demand for coffee has risen, and biologists have begun to explore the impacts human management practices have on the native plants and animals in forests where coffee is grown (70). Much of this research has taken place in Latin America where studies have consistently demonstrated that shade-grown coffee can contribute to the conservation of biodiversity (71).
Ethiopia is the original and only natural reservoir of wild Coffea arabica (Eudicots: Rubiaceae), widely recognized as the source of the world’s most consumed and best coffee (a second species, Coffea canephora [Eudicots: Rubiaceae], native to central and west Africa is used in lower-grade coffee blends) (72). Ethiopia is also the world’s fifth largest producer of coffee, which accounts for 34% of export revenues and supports 15 million smallholder farmers (73). Coffee has traditionally grown naturally in the forests of southwest Ethiopia, although these forests are increasingly being cleared or modified to boost coffee production (73). Indeed, despite the long-recognized importance of coffee forests as reservoirs for biodiversity (even in countries where coffee is an introduced species), the decline in shade-grown coffee cropland coupled with the rise in monoculture sun farms globally over the last few decades has spelled disaster for many species and peoples that rely on the forests for their livelihoods (74). Given the increasing global demand for coffee (75), it is critical to understand how coffee agroforestry impacts plant and animal populations everywhere coffee is grown.
As the natural reservoir of C. arabica and its genetic diversity, the montane forests of southwest Ethiopia provide a unique laboratory within which to compare the ecology and conservation biology of coffee forests under both natural and human-modified conditions. Compared with natural forests containing C. arabica, shade coffee farms in Ethiopia support fewer species of trees (76) and other plants, including orchids (77). These findings are not, in themselves, surprising since some disturbance to the plant community is inevitable when coffee is selectively increased (to boost yield) at the expense of other plants in the same forest.
How might changes in the plant community as a result of shade coffee farming in Ethiopia affect ecosystem processes and services? How might they affect animals in the forest? Two recent studies in the region found that fewer species of insects visited C. arabica flowers in more intensively managed coffee forests than in natural or seminatural coffee forests (78, 79). This apparent reduction in the insect community in more managed settings may adversely impact coffee pollination and production over time (79). Shade coffee farms appear to provide suitable habitat for many bird species in Ethiopia (like elsewhere in the tropics). Indeed, shade coffee farms in Ethiopia support more species of birds than nearby natural forest, although the abundance of two at-risk groups of birds, forest specialists, and understory insectivores, was higher in natural forests (80). While intact forests undoubtedly provide the best habitat for tropical birds, Ethiopian shade-grown coffee may be the most bird-friendly coffee in the world, unsurprising given that coffee is native to the forests of southwest Ethiopia (80, 81). In the case of large mammals, recent research suggests that most species are as likely to occur in managed shade coffee forest systems as in natural coffee forest, an exception being the most intensively managed coffee farms where canopy cover is reduced and many large mammals are absent (82, 83). Large mammals in managed coffee forest do shift their activity patterns toward crepuscularity and nocturnality in contrast to the more diurnal tendencies of these same mammals in natural forest (82). Intriguingly, the berries of C. arabica are consumed by De Brazza’s monkeys (Mammalia: Cercopithecidae: Cercopithecus neglectus) (84) and African civets (Mammalia: Viverridae: Civettictis civetta) (85), suggesting that these secretive species may play roles as seed dispersers in natural coffee forests. In summary, while most large mammals and birds studied appear capable of adapting to lightly managed coffee forests, the trend toward management intensification across Ethiopia’s coffee forest region is concerning, given its potential for adversely impacting biodiversity, pollination services, and coffee yields.
Because the cultivars of C. arabica grown worldwide originate from a very narrow genetic base stock originally from southwest Ethiopia, the larger diversity growing naturally in the region’s forests is of paramount conservation and economic importance as climate change and biotic hazards increasingly wreak havoc on existing cultivars (86). In recent modeling studies, researchers found that climate change could render most of Ethiopia’s current coffee-growing areas unsuitable for coffee farming in the coming decades, unless mitigating strategies like relocation and forest conservation or reestablishment are undertaken soon (87, 88). The time to act to further study and safeguard the wild progenitors of the world’s favorite coffee species and Ethiopia’s coffee-growing tradition is now.
Community Conservation, Fortress Conservation, and Nature-Based Solutions.
A fundamental problem of both conservation biology and sustainable development is how to best protect nature (and natural processes) in perpetuity for the mutual benefit of people and nature in a world that has become increasingly human dominated (89). Researchers have combed the world (and historical records) searching for possible answers to this seemingly intractable problem, while governments and international agencies have adopted a variety of measures to try to stem the loss or degradation of the world’s remaining wild places.
Fortress conservation initiatives, which strictly limit human use of protected areas, are among the most common approaches to protecting biodiversity (90). Although largely successful in North America and Europe, fortress conservation has met with more opposition and less success in developing countries where funding to patrol reserves is often limited, and local economies frequently rely directly on resources from protected areas (91). In recent decades, interest in conservation approaches that involve the participation of local communities has grown (92). Examples of long-held traditions of nature conservation can be found today on all continents except Antarctica (93). They are typically maintained through traditional methods of community conservation that do not require federal or regional government oversight. Although often small in size, these sites are important for landscape connectivity and collectively have important conservation value (93).
With its rapidly growing population and shrinking natural habitats, Ethiopia might seem an unlikely place to find successful conservation traditions. Yet, Africa’s oldest recorded example of official conservation efforts comes from Ethiopia. Dismayed by forest loss on Wuchacha Mountain, Emperor Zera Yacob (1434–1468) ordered the planting of young African juniper (Pinophyta: Cupressaceae: Juniperus procera), the species that once dominated the landscape of the Ethiopian Highlands, creating Menagesha Forest, which continues to be conserved to this day as the Menagesha-Suba National Forest Priority Area (7) (Fig. 1).
The church forests of the northern Ethiopian Highlands provide a striking example of long-standing community conservation efforts that have helped to protect biodiversity in a densely populated region. Only ~1,000 km2 of high forest remains in this region, much of it in the form of small fragments surrounding 35,000 Ethiopian Orthodox Tewahedo churches and monasteries. Each church manages its forest autonomously and protection ranges from nonexistent to walls and patrols by paid guards (94). While individual church forests tend to have low species richness, and often include some exotic species (95), collectively, they contain ≥148 of the 270 indigenous tree species found in tropical northeast Africa (96). In comparison, even the largest continuous forests remaining in the northern Highlands contain no more than 43–66 species (95, 96).
Traditionally animist peoples of the southwestern Ethiopian Highlands, including the Gamo, Gedeo, Gofa, Konso, Sheka, and Sidama, have also long preserved sacred forests amid agricultural landscapes. Their resilient agricultural systems depend on the ecosystem services provided by the sacred forests, important reservoirs of indigenous tree species and other biodiversity in a densely populated region (97, 98). In addition to the religious and spiritual rationales for conserving the sacred forests of southwest Ethiopia and the church forests of northern Ethiopia, people value these forests for their ethnobotanical uses. Traditional medicines are produced from many sacred and church forest plants and are crucial to people who prefer traditional treatments or lack access to western medicines (99, 100). Overall, like other sacred forest sites worldwide, the church forests of northern Ethiopia and sacred forests of southwest Ethiopia are collectively crucial not only to the conservation of biodiversity at the regional level but also to the daily lives of the peoples conserving them (93).
Not all community conservation traditions are small in scale. A remarkably resilient community-based conservation effort, the Qero system, in the northern Highlands of Ethiopia, has successfully managed a large area of Afroalpine grassland at Guassa over the past 400 y (101). Locals in the area developed stringent rules for the grassland’s use and penalties for violating them that have evolved to keep protections in place over the centuries (101, 102). Without designation as a national park and with limited outside aid, the grassland at Guassa is among the largest, most ecologically intact grasslands in the Ethiopian Highlands (38, 102).
Contrary to conventional wisdom which argues that the only effective means of sustainably regulating the use of common resources is through centralized government or private control, the examples of successful community conservation traditions in Ethiopia lasting centuries with little outside input highlight the essential value of local initiative and participation in the conservation of nature (102, 103). Unfortunately, traditional conservation systems in Ethiopia, like elsewhere (93), today face growing threats—including unsustainable population growth, development, political instability, and climate change. For example, at Guassa, recent road construction projects have resulted in wild animal collisions and soil erosion. Furthermore, ~500 hectares of Guassa’s alpine grassland were recently burned during fighting associated with Ethiopia’s civil war ( SI Appendix, Fig. S3). Continued study at Guassa and other traditional conservation areas would reveal how Ethiopia's long-standing community conservation systems cope with the unprecedented array of threats they face.
Beginning with Emperor Zera Yacob’s edict to create and protect Menagesha Forest, fortress conservation has also long played a role in Ethiopian conservation strategies (7). Emperor Haile Selassie established Ethiopia’s first seven national parks between 1958 and 1974, and the protected area system has continued to expand since. Today, there are >135 protected areas, collectively covering 14% of Ethiopia’s landmass and containing much of its biodiversity (Fig. 1 and SI Appendix, Table S1). Funding and/or oversight for this system comes from collaborations between the Ethiopian federal government, International Union for the Conservation of Nature, United Nations Educational, Scientific and Cultural Organization (UNESCO), non-governmental organizations, and regional and local stakeholders, although obtaining sufficient financing remains a perpetual issue (104). Ethiopia’s two most venerated national parks, the SMNP and the BMNP, hold some of its most iconic wildlife and have attracted much international funding and research by foreign and Ethiopian scientists alike. The SMNP is a UNESCO World Heritage Site, and the Ethiopian government and stakeholders have made it a priority to achieve this same designation for the BMNP. Still, threats from village expansion, agricultural encroachment, livestock overgrazing, deforestation, and fire plague these high-profile, legally protected key biodiversity areas (105, 106), problems shared by many other protected areas in Ethiopia. Encouragingly, most Ethiopians surveyed living near protected areas regard them positively (107). Furthermore, households near national parks have higher average incomes than those further away (108). Still, only continued long-term monitoring and study of biodiversity in Ethiopia's protected areas will establish the extent to which fortress conservation can succeed there.
Given the increasing pressures on Ethiopia’s protected areas and the long history of community-based conservation in some regions, the increasingly popular conservation approach of nature-based solutions may hold promise for tackling some of Ethiopia’s conservation challenges. In some ways an intermediate approach between fortress and community conservation, nature-based solutions involve solving challenges facing human society by working with and conserving nature (109). These solutions are usually most effective when implemented through collaboration between governmental officials, scientific experts, and local people (109). For example, in the southern Ethiopian Highlands, bamboo is valued not only by local people for constructing homes, household goods, and fences but also by Bale monkeys and other bamboo forest–dwelling animals as their primary food source or habitat (22). The unsustainable harvesting of bamboo in this region thus threatens both human livelihoods and wildlife. Mekonnen and colleagues (22) recently advocated for a nature-based solution to this problem through the restoration of declining montane bamboo forest habitat, simultaneously benefiting biodiversity and contributing toward meeting several United Nations Sustainable Development Goals. Moving forward, creative nature-based solutions have the potential to be an important component of conservation strategies in Ethiopia.
Perspectives for Future Research.
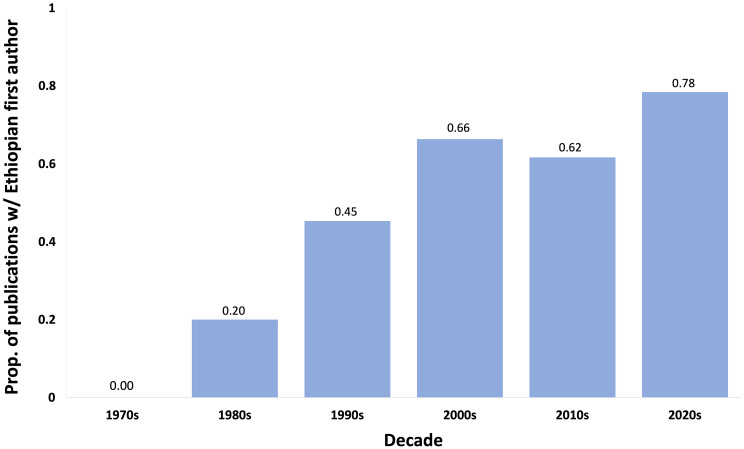
The potential for biological research—be it ecology, evolution, systematics, or conservation biology—in Ethiopia is enormous. Few countries containing such high levels of endemism have received as little study as Ethiopia. With its university systems expanding and the number of PhD-level scientists trained at home and abroad growing rapidly (32), biological research is occurring at an unprecedented pace in Ethiopia today. Indeed, a transition from a research agenda carried out by foreign scientists to primarily Ethiopian-led research has occurred over the past half century (Fig. 2). We conclude our overview of biodiversity research in Ethiopia by suggesting directions for future research that are especially promising.
Fig. 2.
Proportion of peer-reviewed journal articles (Web of Science search, September 15, 2022) on extant Ethiopian biodiversity (n=745) first-authored by Ethiopian scientists by decade between the 1970s and 2020s. Title or author keywords search terms: “Ethiopia* AND ecology OR mammal* OR bird* OR plant* OR insect* OR reptile* OR fish* OR forest* OR invertebrate* OR new species OR amphibian* OR biodiversity”. Articles concerning extinct Ethiopian species and solely on crop plants or domestic animals were excluded from the analysis. Data from which this graph was generated are available in the accompanying data file “Data for Fig 2.xlsx”.
Climate Change and Evolution.
Ethiopia’s extensive elevation gradients generate incredible climatic diversity (110), conditions well suited to studies of local adaptation, dispersal, and the evolutionary constraints on trait expression in widely distributed plants, although these research opportunities remain underutilized (111, 112). In Ethiopia, populations of the widely studied model plant species, Arabidopsis thaliana (Eudicots: Brassicaceae), occupy a climatically unique niche in its global distribution. These populations are genetically distinct from other global populations, and strong signals of local adaptation within mountain ranges could be valuable to understanding general plant adaptations across elevation gradients that ultimately could be used for crop improvements (111).
As the continent most reliant on rain-fed agriculture by small-scale subsistence farmers for its food production, Africa is regarded as especially susceptible to the impacts of climate change (113). Ethiopia is considered to be one of the African countries most at risk to climate change because of its large population and devastating history of food insecurity (114). Computer modeling studies suggest that the areas over which a) the four major cereal crops (teff, maize, sorghum, and barley) on which Ethiopians are most dependent and b) coffee, Ethiopia’s most important agricultural commodity, occur will be significantly reduced by climate change (87, 88, 114). Furthermore, the shift toward increasingly managed coffee forests which are more reliant on honeybees than natural pollinators will likely lead to reduced coffee production in Ethiopia given that global pollination services by managed honeybees are anticipated to decline with climate change (78). These empirically based predictions are consistent with concerns raised by the World Bank that the economic and social impacts of climate change on Ethiopia will be immense (115).
Little research has been carried out on the impacts of climate change on Ethiopia’s biodiversity. A simulation model of the projected impacts of climate change on geladas concluded that the minimum altitude they can occupy will increase by 500 meters for every 2°C rise in mean temperature (116). Another study reported high rates of dieback in the two most dominant tree species in the northern Ethiopian Highlands – the African juniper and the olive (Eudicots: Oleaceae: Olea europaea subsp. cuspidata) – concluding that the climate change mitigation (i.e., carbon sequestration) potential of the remnant dry forests in the region will decline rapidly without urgent rehabilitation efforts and enrichment planting (117).
Afroalpine plants in Ethiopia’s high mountains also face high risk of habitat loss and genetic depauperation. For example, a recent ecological niche modeling study on the habitat suitability of the Ethiopian endemic giant lobelia, under two climate models and four emission scenarios across the Ethiopian Highlands, revealed significant range reduction and loss of genetic diversity. The study reported that by 2080, only 3.4% of the giant lobelia’s habitat will remain suitable, resulting in an 82% loss of its genetic diversity (118). Another study, based on novel genome skimming data and published matrices comprising ~100 flowering plant species endemic to the Afroalpine habitats of East Africa, confirms that the Ethiopian Highlands house the region’s oldest plant lineages despite ~80% of the species having likely originated during the last 5 My. This finding points to the importance of the Ethiopian mountains as vital refugia for the oldest Afroalpine plant lineages (119). Despite these exciting recent studies, large-scale studies of the impacts of climatic change on plant and animal populations over the long term (120) are much needed in Ethiopia.
Ecology and Conservation in the Anthropocene.
In addition to further research on the biological impacts of climate change, there is a need for more natural history research on Ethiopian biodiversity, including ramping up efforts at taxonomic assessment and systematics for arthropods, bryophytes, lichens, and other poorly known groupings before it is too late (121 –124). These efforts would enable us to better monitor spatial and temporal changes in species and communities in response to natural and human-caused changes in the environment (125). Furthermore, despite well-documented high species richness (21), birds remain little studied throughout much of Ethiopia. The organisms in Ethiopia's aquatic ecosystems have been similarly understudied. Moreover, none of the wildlife of the Horn of Africa—aside from baboons (49, 126)—has received long-term study (8). Ethiopia’s elephants (Mammalia: Elephantidae: Loxodonta africana) must be considered a research and conservation priority. Less than 1,000 individuals remain in six populations scattered across southern Ethiopia, and little else is known about these elephants, which are threatened by habitat destruction and poaching (127). Furthermore, while notable research has been carried out on species-level disease ecology in Ethiopian wolves (52, 53) and geladas (43, 51), no attempts have yet been made at achieving a more comprehensive understanding of wildlife disease ecology through study at the community level (128).
Biological invasions pose a significant threat to Ethiopia’s biodiversity but, like in much of Africa (129), have been little studied (69). In recent decades, several alien plants are known to have become invasive over large areas—e.g., mesquite (Eudicots: Fabaceae: Prosopis juliflora) in the Afar Region and water hyacinth (Eudicots: Pontederiaceae: Eichhornia crassipes) in Lake Tana—reducing the ecosystem service values of the habitats they invaded (130, 131). Invasive alien insects and mites are also a growing issue—potential threats to agriculture and biodiversity alike—although the true scale of the problem is largely unknown (132). For example, although over 1,300 spider mite (Arachnida: Tetranychidae) species have been catalogued worldwide, including 28 in nearby Yemen and 21 in bordering Kenya, only five have been identified to date in Ethiopia, four of them alien crop pests (133). The invasive alien fall armyworm (Insecta: Noctuidae: Spodoptera frugiperda), introduced to Ethiopia in 2017, has spread to six maize producing regions of the country where it feeds upon a wide variety of plants. Its potential impacts on agriculture and biodiversity are substantial (134). Greater funding, expertise, and research related to current and potential invasive species must be considered priorities for Ethiopia.
Ethiopia’s coffee forests are well studied, but the extent to which its other agroforestry systems also contribute to biodiversity conservation remains less known. For example, while ensete (Eudicots: Musaceae: Ensete ventricosum, a banana-like plant only domesticated and grown for food in Ethiopia) agroforestry supports many native woody species, including some of conservation value (135), the extent to which it supports other native flora and fauna remains to be studied. Additional research is also needed on the resilience of Ethiopia’s ancient community conservation systems and the biodiversity they harbor (94, 96, 101) in the face of mounting challenges—from civil strife to rapid human population growth and infrastructure development. Although logistical challenges abound, there are many opportunities to extend and complement previous research on the biodiversity of Ethiopia, and we hope that this review will prove a useful resource for those who will carry out this important work.
Implications for Africa and the Tropical World.
Beyond Ethiopia, there are lessons from this review that we believe can be applied more broadly to Africa and the wider tropical world. Like a canary in a coal mine, Ethiopia offers an early indicator of what the future has in store for tropical Africa. Because of its unique geography, topography, and geology, Ethiopia is an ideal testing ground not only for evolutionary and ecological theory but also for studying the effects of global change (136). The high elevation and tropical geography of the Ethiopian Highlands shape strong seasonal rainfall and temperature patterns, but like other alpine regions across the globe (137), this abiotic seasonality is sensitive to climate change (136). Ethiopia’s several decades-long studies of social mammals in particular offer exciting opportunities to explore the direct and indirect effects of climate variability on socially complex animals, a crucial topic that is only just now beginning to receive scientific attention (138, 139). The lessons we learn from studying how Ethiopia’s biodiversity adapts (or fails to adapt) to climate variability in the near future offers a window into how the rest of sub-Saharan Africa’s biodiversity may respond to a changing climate in the future.
Our review also demonstrates the benefits to both science and society of promoting scientific development in one of the world’s poorest but fastest-growing countries. Much of the recent research discussed here was led by Ethiopian scientists, many of them newly minted PhDs with bright scientific futures. Increasing investment in higher education by the Ethiopian government and by European countries in training Ethiopian scientists has facilitated this burst in scientific training and output from Ethiopia (32), although training of female scientists at the PhD level has lagged far behind that of male scientists and is a priority area to be addressed (140). Ethiopian scientists face various challenges shared by scientists in many other African countries that are not experienced by most of their counterparts in high-income countries. These include lower pay, poorly equipped laboratories and libraries, inconsistent internet, and many others (32, 141). Under these circumstances, successfully competing for large international sources of grant money is challenging. Support from collaborators and institutions in high-income countries with greater access to funding and research infrastructure will continue to contribute to science development in Ethiopia until greater scientific parity with high-income countries has been reached (32). Also important will be the development of more collaborations between Ethiopian scientists and their counterparts in other African countries (142). Despite the obstacles that remain, the recent rapid growth of scientific expertise in Ethiopia should be celebrated and serve as a road map for other countries not yet on the same trajectory. Investment in higher education, especially in science, has been a key driver of socioeconomic development in East Asia (143), and we hope will pay similar dividends for Ethiopia and other developing countries that follow this path (32).
Last, the examples summarized here of successful community-led conservation efforts from Ethiopia add to a small but growing list of effective solutions to the challenges inherent in protecting and managing common-pool resources, which are especially pressing problems in biodiversity-rich, tropical locations today (103). Across the tropics, fortress conservation initiatives, funded by centralized governments or large conservation organizations, while well intentioned, have often failed to avert biodiversity loss. Indeed, a recent meta-analysis found that half of 60 well-studied protected areas in the tropics had experienced major declines in biodiversity (among 31 animal and plant guilds) over the past several decades (144). While no single strategy is a panacea for all conservation challenges, the examples of successful conservation initiatives from Ethiopia, which range from “fortresses” erected by past emperors to community-led efforts, offer important lessons to other tropical nations in how to successfully employ a mixture of conservation strategies to combat biodiversity loss.
Conclusion
Ethiopia is a high-biodiversity country and contains two major centers of endemism, the mesic Roof of Africa and the arid Horn of Africa. However, with its large and rapidly growing human and livestock populations, widespread poverty, civil strife, complex land policy issues, and mounting pressure to exploit its natural resources for economic development, there are few countries where the conservation of biodiversity poses a greater challenge than Ethiopia. Against this complex backdrop, several key areas of research on Ethiopia’s biodiversity—particularly the behavioral ecology of its large social mammals, the ecology and conservation of its unique coffee forests, and successful approaches to conservation in Ethiopia (some originating several hundred years ago)—have broadened our understanding of nature and its conservation in Africa. Ethiopia’s scientific capacity has burgeoned over the past few decades, and the vast majority of research on its biodiversity is now being produced by Ethiopian scientists. There are many important areas for future research in evolutionary biology, ecology, systematics, and conservation in Ethiopia. These include 1) the long-term impacts of climate change on biodiversity across Ethiopia’s wide array of elevational gradients and ecosystems; 2) taxonomic assessments for understudied groups including invertebrates, bryophytes, and lichens; 3) the behavior, ecology, and conservation of the little-known wildlife of the Horn of Africa region and Ethiopia’s declining and fragmented elephant populations; 4) the threats to biodiversity and agriculture from biological invasions; 5) community-level studies of wildlife disease ecology; 6) the biodiversity conservation potential of ensete and other little-studied agroforestry systems; and 7) the impacts of population growth, civil conflict, and infrastructure development on Ethiopia’s long-standing community conservation systems.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Acknowledgments
P.J.F. and N.N. thank the U.S.-Norway Fulbright Foundation, California State University Fullerton, University of Oslo, Pittsburgh Zoo, and San Diego Zoo for their support. S.D. acknowledges support from the Carlsberg Foundation, University of Marburg, University of Oslo, and Addis Ababa University. J.T.K. thanks the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant (agreement no. 754513) and the Aarhus University Research Foundation for their support. N.C.S. thanks the Centre for Ecological and Evolutionary Synthesis (CEES) at the University of Oslo for providing an excellent platform for training a large number of students from eastern Africa—which has been made possible through generous Norwegian funding over many years. We also thank the many senior and early-stage scientists from Ethiopia and abroad whose research inspired this review. Berhane Asfaw, Colin Chapman, Gisela Fashing, Norman Fashing, Antoine Souron, and two anonymous reviewers kindly provided helpful comments on earlier drafts of this manuscript.
Author contributions
P.J.F., N.N., and N.C.S. conceived the idea for the paper; and P.J.F., N.N., S.D., A.G., A.A., A.M., N.O.N., J.T.K., and N.C.S. contributed to the writing of the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All data are available in the main text and SI Appendix . Map files for Fig. 1 are available upon request.
Supporting Information
References
- 1. Brooks T., et al. , Toward a blueprint for conservation in Africa. BioScience 51 , 613–624 (2001). [Google Scholar]
- 2. Phillipson D. W., Foundations of an African Civilisation: Aksum and the Northern Horn 1000 BC-AD 1300 (James Currey, Oxford, 2014), p. 304. [Google Scholar]
- 3. Mercier J., Lepage C., Lalibela: Christan Art of Ethiopia, the Monolithic Churches and their Treasures (Paul Holberton Publishing, London, 2012), p. 320. [Google Scholar]
- 4. White T. D., et al. , Ardipithecus ramidus and the paleobiology of early hominids. Science 326 , 64–86 (2009). [PubMed] [Google Scholar]
- 5. Haile-Selassie Y., Melillo S. M., Vazzana A., Benazzi S., Ryan T. M., A 3.8-million-year-old hominin cranium from Woranso-Mille, Ethiopia. Nature 573 , 214–219 (2019). [DOI] [PubMed] [Google Scholar]
- 6. Asfaw B., et al. , Australopithecus garhi: A new species of early hominid from Ethiopia. Science 284 , 629–635 (1999). [DOI] [PubMed] [Google Scholar]
- 7. Williams S., Pol J. L. V., Spawls S., Shimelis A., Kelbessa E., “Ethiopian Highlands” in Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions, Mittermeier R. A., et al., Eds. (Conservation International, Washington, D. C., 2005), pp. 262–273. [Google Scholar]
- 8. Thulin M., “Horn of Africa” in Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions, Mittermeier R. A., et al., Eds. (Conservation International, Washington, D.C., 2005), pp. 276–283. [Google Scholar]
- 9. Friis I., Demissew S., van Breugel P., Atlas of the Potential Vegetation of Ethiopia (Royal Danish Academy of Sciences and Letters, Copenhagen, 2010), p. 315. [Google Scholar]
- 10. Kingdon J., Island Africa (Princeton University Press, Princeton, NJ, 1990), p. 287. [Google Scholar]
- 11. Zewde B., A century of Ethiopian historiography. J. Ethiop. Stud. 33 , 1–26 (2000). [Google Scholar]
- 12. Egziabher T. B. G., Issues in the development of botany as a science in Ethiopia and the Ethiopian Flora Project. Symb. Bot. Ups. 26 , 1–8 (1986). [Google Scholar]
- 13. Kidane Y. O., Beierkuhnlein C., Vegetation dynamics, and land use and land cover change in the Bale Mountains, Ethiopia. Environ. Monit. Assess. 184 , 7473–7489 (2012). [DOI] [PubMed] [Google Scholar]
- 14. Billi P., Landscapes and Landforms of Ethiopia (Springer, Utrecht, Netherlands, 2015), p. 389. [Google Scholar]
- 15. Yalden D. W., The extent of high ground in Ethiopia compared to the rest of Africa. SINET Ethiop. J. Sci. 6 , 35–40 (1983). [Google Scholar]
- 16. Friis I., Thulin M., Adsersen H., Burger A.-M., Patterns of plant diversity and endemism in the Horn of Africa. Biol. Skr. 55 , 289–314 (2005). [Google Scholar]
- 17. Butler R. A., The top 10 most biodiverse countries: What are the world's most biodiverse countries? https://news.mongabay.com/2016/05/top-10-biodiverse countries (2016) . Accessed 22 October 2022.
- 18. Hedberg I., Edwards S., Eds., The Flora of Ethiopia and Eritrea Volumes 1–8 (University of Uppsala, Uppsala, Sweden, 1986–2009). [Google Scholar]
- 19. Kelbessa E., Demissew S., Diversity of vascular plant taxa of the flora of Ethiopia and Eritrea. Ethiop. J. Biol. Sci. 13 , 37–45 (2014). [Google Scholar]
- 20. Demissew S., Friis I., Weber O., Diversity and endemism of the flora of Ethiopia and Eritrea: State of knowledge and future perspectives. Rendiconti Lincei. Scienze Fisiche e Naturali 32 , 675–697 (2021). [Google Scholar]
- 21. Ash J., Atkins J., Birds of Ethiopia and Eritrea (Bloomsbury, London, 2009), p. 456. [Google Scholar]
- 22. Mekonnen A., Fashing P. J., Chapman C. A., Venkataraman V. V., Stenseth N. C., The value of flagship and umbrella species for restoration and sustainable development: Bale monkeys and bamboo forest in Ethiopia. J. Nat. Conserv. 65 , 126117 (2022). [Google Scholar]
- 23. IUCN, The IUCN Red List of Threatened Species. Version 2016-1 (International Union for the Conservation of Nature, 2016), www.iucnredlist.org. Accessed 1 July 2016. [Google Scholar]
- 24. Froese R., Pauly D., FishBase (World Wide Web electronic publication). (2022). Accessed 25 October 2022.
- 25. Mengistu A. A., Nagel P., Getahun A., Saber S. A., Loader S. P., Updated review of amphibian diversity, distribution and conservation in Ethiopia. Ethiop. J. Biol. Sci. 12 , 81–116 (2013). [Google Scholar]
- 26. EBI, Ethiopia’s Fifth National Report to the Convention on Biological Diversity (Ethiopian Biodiversity Instit; ute, Government of Ethiopia, Addis Ababa, Ethiopia, 2014). [Google Scholar]
- 27. U.N., World Population Prospects: The 2022 Revision. (United Nations Department of Economic and Social Affairs, Population Division, 2022). [Google Scholar]
- 28. FAO, Global Forest Resources Assessment 2020: Main report (Food and Agriculture Organization of the United Nations, Rome, 2020). [Google Scholar]
- 29. Tolessa T., Senbete F., Kidane M., The impact of land use/land cover change on ecosystem services in the central highlands of Ethiopia. Ecosyst. Serv. 23 , 47–54 (2017). [Google Scholar]
- 30. Nyssen J., et al. , Environmental conditions and human drivers for changes to north Ethiopian mountain landscapes over 145 years. Sci. Total Environ. 485 , 164–179 (2014). [DOI] [PubMed] [Google Scholar]
- 31. Soboka T. E., “Post-Cold War Ethiopian land policy and state power in land commercialisation” in African Land Reform Under Economic Liberalisation: States, Chiefs, and Rural Communities, Takeuchi S., Ed. (Springer, Singapore, 2022), pp. 153–180. [Google Scholar]
- 32. Atickem A., et al. , Build science in Africa. Nature 570 , 297–300 (2019). [DOI] [PubMed] [Google Scholar]
- 33. Kummer H., Social Organization of Hamadryas Baboons (University of Chicago Press, Chicago, IL, 1968), p. 189. [Google Scholar]
- 34. Dunbar R., Dunbar P., Social Dynamics of Gelada Baboons (Karger, Basel, 1975), p. 158. [PubMed] [Google Scholar]
- 35. Nievergelt B., Ibexes in an African Environment (Springer-Verlag, New York, 1981), p. 202. [Google Scholar]
- 36. Groves C. P., Primate Taxonomy (Smithsonian Institution Press, Washington, D.C., 2001). [Google Scholar]
- 37. Swedell L., Hailemeskel G., Schrier A., Composition and seasonality of diet in wild hamadryas baboons: Preliminary findings from Filoha. Folia Primatol. 79 , 476–490 (2008). [DOI] [PubMed] [Google Scholar]
- 38. Fashing P. J., Nguyen N., Venkataraman V. V., Kerby J. T., Gelada feeding ecology in an intact ecosystem at Guassa, Ethiopia: Variability over time and implications for theropith and hominin dietary evolution. Am. J. Phys. Anthropol. 155 , 1–16 (2014). [DOI] [PubMed] [Google Scholar]
- 39. Mekonnen A., Bekele A., Fashing P. J., Hemson G., Atickem A., Diet, activity patterns, and ranging ecology of the Bale monkey (Chlorocebus djamdjamensis) in Odobullu Forest, Ethiopia. Int. J. Primatol. 31 , 339–362 (2010). [Google Scholar]
- 40. Chiou K. L., et al. , Genomic signatures of high-altitude adaptation and chromosomal polymorphism in geladas. Nat. Ecol. Evol. 6 , 630–643 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marino J., Threatened Ethiopian wolves persist in small isolated Afroalpine enclaves. Oryx 37 , 62–71 (2003). [Google Scholar]
- 42. Clutton-Brock T., Sheldon B. C., Individuals and populations: The role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 25 , 562–573 (2010). [DOI] [PubMed] [Google Scholar]
- 43. Nguyen N., et al. , Fitness impacts of tapeworm parasitism on wild gelada monkeys at Guassa, Ethiopia. Am. J. Primatol. 77 , 579–594 (2015). [DOI] [PubMed] [Google Scholar]
- 44. Roberts E. K., Lu A., Bergman T. J., Beehner J. C., A Bruce effect in wild geladas. Science 335 , 1222–1225 (2012). [DOI] [PubMed] [Google Scholar]
- 45. Amann A. L., Pines M., Swedell L., Contexts and consequences of takeovers in hamadryas baboons: Female parity, reproductive state, and observational evidence of pregnancy loss. Am. J. Primatol. 79 , e22649 (2017). [DOI] [PubMed] [Google Scholar]
- 46. Grueter C. C., Qi X., Li B., Li M., Multilevel societies. Curr. Biol. 27 , R984–R986 (2017). [DOI] [PubMed] [Google Scholar]
- 47. Swedell L., Plummer T., Social evolution in Plio-Pleistocene hominins: Insights from hamadryas baboons and paleoecology. J. Hum. Evol. 137 , 102667 (2019). [DOI] [PubMed] [Google Scholar]
- 48. Beehner J. C., Bergman T. J., Infant mortality following male takeovers in wild geladas. Am. J. Primatol. 70 , 1152–1159 (2008). [DOI] [PubMed] [Google Scholar]
- 49. Swedell L., Leedom L., Saunders J., Pines M., Sexual conflict in a polygynous primate: Costs and benefits of a male-imposed mating system. Behav. Ecol. Sociobiol. 68 , 263–273 (2014). [Google Scholar]
- 50. Roberts E. K., Lu A., Bergman T. J., Beehner J. C., Female reproductive parameters in wild geladas (Theropithecus gelada). Int. J. Primatol. 38 , 1–20 (2017). [Google Scholar]
- 51. Schneider-Crease I., Griffin R. H., Gomery M. A., Bergman T. J., Beehner J. C., High mortality associated with tapeworm parasitism in geladas (Theropithecus gelada) in the Simien Mountains National Park, Ethiopia. Am. J. Primatol. 79 , e22684 (2017). [DOI] [PubMed] [Google Scholar]
- 52. Marino J., Sillero-Zubiri C., Macdonald D. W., Trends, dynamics and resilience of an Ethiopian wolf population. Anim. Conserv. 9 , 49–58 (2006). [Google Scholar]
- 53. Gordon C., et al. , Canine distemper in endangered Ethiopian wolves. Emerging Infect. Dis. 21 , 824–832 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Randall D., et al. , Rabies in endangered Ethiopian wolves. Emerging Infect. Dis. 10 , 2214–2217 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Randall D. A., et al. , An integrated disease management strategy for the control of rabies in Ethiopian wolves. Biol. Conserv. 131 , 151–162 (2006). [Google Scholar]
- 56. Mekonnen A., et al. , Dietary flexibility of Bale monkeys (Chlorocebus djamdjamensis) in southern Ethiopia: Effects of habitat degradation and life in fragments. BMC Ecol. 18 , 4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mekonnen A., et al. , Impacts of habitat loss and fragmentation on the activity budget, ranging ecology and habitat use of Bale monkeys (Chlorocebus djamdjamensis) in the southern Ethiopian Highlands. Am. J. Primatol., 79 , e22644 (2017). [DOI] [PubMed] [Google Scholar]
- 58. Tesfaye D., Fashing P. J., Meshesha A. A., Bekele A., Stenseth N. C., Feeding ecology of the Omo River guereza (Colobus guereza guereza) in habitats with varying levels of fragmentation and disturbance in the southern Ethiopian Highlands. Int. J. Primatol. 42 , 64–88 (2021). [Google Scholar]
- 59. Tesfaye D., Fashing P. J., Bekele A., Mekonnen A., Atickem A., Ecological flexibility in Boutourlini’s blue monkeys (Cercopithecus mitis boutourlinii) in Jibat Forest, Ethiopia: A comparison of habitat use, ranging behavior, and diet in intact and fragmented forest. Int. J. Primatol. 34 , 615–640 (2013). [Google Scholar]
- 60. Gebremedhin B., et al. , DNA metabarcoding reveals diet overlap between the endangered walia ibex and domestic goats - implications for conservation. PLoS One 11 , e0159133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Atickem A., Loe L. E., Livestock-wildlife conflicts in the Ethiopian highlands: Assessing the dietary and spatial overlap between mountain nyala and cattle. Afr. J. Ecol. 52 , 343–351 (2014). [Google Scholar]
- 62. Tamrat M., et al. , Swayne’s hartebeest in Ethiopia: Population estimate, genetic variability and competition with livestock. Oryx 56 , 336–344 (2022). [Google Scholar]
- 63. Gebremedhin B., et al. , Quest for new space for restricted range mammals: The case of the endangered Walia ibex. Front. Ecol. Evol. 9 , 611632 (2021). [Google Scholar]
- 64. Atickem A., et al. , Estimating population size and habitat suitability for mountain nyala in areas with different protection status. Anim. Conserv. 14 , 409–418 (2011). [Google Scholar]
- 65. Atickem A., et al. , Population genetic structure and connectivity in the endangered Ethiopian Mountain Nyala (Tragelaphus buxtoni): Recommending dispersal corridors for future conservation. Conserv. Genet. 14 , 427–438 (2013). [Google Scholar]
- 66. Rueness E. K., et al. , The cryptic African wolf: Canis aureus lupaster is not a golden jackal and is not endemic to Egypt. PLoS One 6 , e16385 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gutema T. M., et al. , Competition between sympatric wolf taxa: An example involving African and Ethiopian wolves. R. Soc. Open Sci. 5 , 172207 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gutema T. M., et al. , Foraging ecology of African wolves (Canis lupaster) and its implications for the conservation of Ethiopian wolves (Canis simensis). R. Soc. Open Sci. 6 , 190772 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gebrehiwot K., Demissew S., Trends in plant ecology research in Ethiopia (1969–2019): Systematic analysis. Bothalia 52 , 1–16 (2022). [Google Scholar]
- 70. Rice R., Coffee production in a time of crisis: Social and environmental connections. SAIS Rev. 23 , 221–245 (2003). [Google Scholar]
- 71. De Beenhouwer M., Aerts R., Honnay O., A global meta-analysis of the biodiversity and ecosystem service benefits of coffee and cacao agroforestry. Agric. Ecosyst. Environ. 175 , 1–7 (2013). [Google Scholar]
- 72. Teketay D., History, botany and ecological requirements of coffee. Walia 20 , 28–50 (1999). [Google Scholar]
- 73. Davis A. P., et al. , Coffee Atlas of Ethiopia (Kew, Royal Botanic Gardens, 2018), p. 59. [Google Scholar]
- 74. Jha S., et al. , Shade coffee: Update on a disappearing refuge for biodiversity. BioScience 64 , 416–428 (2014). [Google Scholar]
- 75. ICO, Historical Data on the Global Coffee Trade (International Coffee Organization, 2022).(2022). http://ico.org/. Accessed 15 September 2022. [Google Scholar]
- 76. Senbeta F., Denich M., Effects of wild coffee management on species diversity in the Afromontane rainforests of Ethiopia. For. Ecol. Manage. 232 , 68–74 (2006). [Google Scholar]
- 77. Hundera K., et al. , Both forest fragmentation and coffee cultivation negatively affect epiphytic orchid diversity in Ethiopian moist evergreen Afromontane forests. Biol. Conserv. 159 , 285–291 (2013). [Google Scholar]
- 78. Berecha G., Aerts R., Muys B., Honnay O., Fragmentation and management of Ethiopian moist evergreen forest drive compositional shifts of insect communities visiting wild arabica coffee flowers. Environ. Manage. 55 , 373–382 (2015). [DOI] [PubMed] [Google Scholar]
- 79. Geeraert L., et al. , Intensification of Ethiopian coffee agroforestry drives impoverishment of the Arabica coffee flower visiting bee and fly communities. Agrofor. Syst. 93 , 1729–1739 (2019). [Google Scholar]
- 80. Buechley E. R., et al. , Importance of Ethiopian shade coffee farms for forest bird conservation. Biol. Conserv. 188 , 50–60 (2015). [Google Scholar]
- 81. Rodrigues P., et al. , Coffee management and the conservation of forest bird diversity in southwestern Ethiopia. Biol. Conserv. 217 , 131–139 (2018). [Google Scholar]
- 82. Mertens J., Emsens W., Jocqué M., Geeraert L., De Beenhouwer M., From natural forest to coffee agroforest: Implications for communities of large mammals in the Ethiopian highlands. Oryx 54 , 715–722 (2020). [Google Scholar]
- 83. Etana B., et al. , Traditional shade coffee forest systems act as refuges for medium- and large-sized mammals as natural forest dwindles in Ethiopia. Biol. Conserv. 260 , 109219 (2021). [Google Scholar]
- 84. Amare A., “Population ecology of De Brazza’s monkey (Cercopithecus neglectus) in Yayu Biosphere Reserve, Southwestern Ethiopia”, MSc thesis, Addis Ababa University; (2012). [Google Scholar]
- 85. Habtamu T., et al. , Diets of the African civet Civettictis civetta (Schreber, 1778) in selected coffee forest habitat, south-western Ethiopia. Afr. J. Ecol. 55 , 573–579 (2017). [Google Scholar]
- 86. Aerts R., et al. , Genetic variation and risks of introgression in the wild Coffea arabica gene pool in south-western Ethiopian montane rainforests. Evol. Appl. 6 , 243–252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moat J., et al. , Resilience potential of the Ethiopian coffee sector under climate change. Nat. Plants 3 , 17081 (2017). [DOI] [PubMed] [Google Scholar]
- 88. Chemura A., Mudereri B. T., Yalew A. W., Gornott C., Climate change and specialty coffee potential in Ethiopia. Sci. Rep. 11 , 8097 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mace G. M., Whose conservation? Science 345 , 1558–1560 (2014). [DOI] [PubMed] [Google Scholar]
- 90. Miller T. R., Minteer B. A., Malan L.-C., The new conservation debate: The view from practical ethics. Biol. Conserv. 144 , 948–957 (2011). [Google Scholar]
- 91. Oates J. F., Myth and Reality in the Rain Forest: How Conservation Strategies Are Failing in West Africa (University of California Press, Berkeley, CA, 1999). [Google Scholar]
- 92. Buscher B., Whande W., Whims of the winds of time? Emerging trends in biodiversity conservation and protected area management. Conserv. Soc. 5 , 22–43 (2007). [Google Scholar]
- 93. Bhagwat S. A., Rutte C., Sacred groves: Potential for biodiversity management. Front. Ecol. Environ. 4 , 519–524 (2006). [Google Scholar]
- 94. Amare D., Perception of the local community and the willingness to pay to restore church forests: The case of Dera district, northwestern Ethiopia. Forests, Trees and Livelihoods 24 , 173–186 (2016). [Google Scholar]
- 95. Wassie A., Sterck F. J., Bongers F., Species and structural diversity of church forests in a fragmented Ethiopian Highland landscape. J. Veg. Sci. 21 , 938–948 (2010). [Google Scholar]
- 96. Aerts R., et al. , Conservation of the Ethiopian church forests: Threats, opportunities and implications for their management. Sci. Total Environ. 551–552 , 404–414 (2016). [DOI] [PubMed] [Google Scholar]
- 97. Shoddo G. H., The contribution of Gudo forest conservation culture is key to biodiversity conservation: The case of Sheka Zone, southwest Ethiopia. Land Use Policy 113 , 105872 (2022). [Google Scholar]
- 98. Maru Y., Gebrekirstos A., Haile G., Indigenous sacred forests as a tool for climate change mitigation: Lessons from Gedeo community, Southern Ethiopia. J. Sustain. For. 1–28 (2022). [Google Scholar]
- 99. Bongers F., Wassie A., Sterck F. J., Bekele T., Teketay D., Ecological restoration and church forests in northern Ethiopia. J. Drylands 1 , 35–44 (2006). [Google Scholar]
- 100. Maru Y., Gebrekirstos A., Haile G., Indigenous ways of environmental protection in Gedeo community, Southern Ethiopia: A socio-ecological perspective. Cogent Food Agric. 6 , 1766732 (2020). [Google Scholar]
- 101. Ashenafi Z. T., Leader-Williams N., Indigenous common property resource management in the Central Highlands of Ethiopia. Hum. Ecol. 33 , 539–563 (2005). [Google Scholar]
- 102. Steger C., et al. , Knowledge coproduction improves understanding of environmental change in the Ethiopian highlands. Ecol. Soc. 25 , 2 (2020). [Google Scholar]
- 103. Ostrom E., The challenge of common-pool resources. Environment 50 , 8–20 (2008). [Google Scholar]
- 104. Aseres S. A., Sira R. K., Estimating visitors’ willingness to pay for a conservation fund: Sustainable financing approach in protected areas in Ethiopia. Heliyon 6 , e04500 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kidane Y., Stahlmann R., Beierkuhnlein C., Vegetation dynamics, and land use and land cover change in the Bale Mountains, Ethiopia. Environ. Monit. Assess. 184 , 7473–7489 (2012). [DOI] [PubMed] [Google Scholar]
- 106. Jacob M., et al. , Land cover dynamics in the Simien Mountains (Ethiopia), half a century after establishment of the National Park. Reg. Environ. Change 17 , 777–787 (2017). [Google Scholar]
- 107. Tessema M. E., Lilieholm R. J., Ashenafi Z. T., Leader-Williams N., Community attitudes toward wildlife and protected areas in Ethiopia. Soc. Nat. Resour. 23 , 489–506 (2010). [Google Scholar]
- 108. Estifanos T. K., Polyakov M., Pandit R., Hailu A., Burton M., The impact of protected areas on the rural households’ incomes in Ethiopia. Land Use Policy 91 , 104349 (2020). [Google Scholar]
- 109. Seddon N., et al. , Getting the message right on nature-based solutions to climate change. Glob. Change Biol. 27 , 1518–1546 (2021). [DOI] [PubMed] [Google Scholar]
- 110. Van den Hende C., et al. , Analysis of rain-shadows in the Ethiopian Mountains using climatological model data. Clim. Dyn. 56 , 1663–1679 (2021). [Google Scholar]
- 111. Gamba D., The genomics and physiology of abiotic stressors associated with global elevation gradients in Arabidopsis thaliana . bioRxiv [Preprint] (2022). 10.1101/2022.03.22.485410 (Accessed 20 May 2022). [DOI] [Google Scholar]
- 112. Brochmann C., et al. , History and evolution of the afroalpine flora: In the footsteps of Olov Hedberg. Alp. Bot. 132 , 65–87 (2022). [Google Scholar]
- 113. IPCC, Climate change and land: An IPCC special report on climate change, desertification, land degradation, sustainable land managementt, food security, and greenhouse gas fluxes in terrestrial ecosystems. [Shukla P.R., Skea J., Calvo Buendia E., Masson-Delmotte V., Pörtner H.-O., Roberts D. C., Zhai P., Slade R., Connors S., van Diemen R., Ferrat M., Haughey E., Luz S., Neogi S., Pathak M., Petzold J., Portugal Pereira J., Vyas P., Huntley E., Kissick K., Belkacemi M., Malley J., (eds.)] (2019).
- 114. Evangelista P., Young N., Burnett J., How will climate change spatially affect agriculture production in Ethiopia? Case studies of important cereal crops. Clim. Change 119 , 855–873 (2013). [Google Scholar]
- 115. World Bank, Ethiopia (The World Bank, 2021), https://climateknowledgeportal.worldbank.org/country/ethiopia/vulnerability. Accessed 22 October 2022. [Google Scholar]
- 116. Dunbar R. I. M., Impact of global warming on the distribution and survival of the gelada baboon: A modelling approach. Glob. Change Biol. 4 , 293–304 (1998). [Google Scholar]
- 117. Mokria M., Gebrekirstos A., Aynekulu E., Bräuning A., Tree dieback affects climate change mitigation potential of a dry afromontane forest in northern Ethiopia. For. Ecol. Manage. 344 , 73–83 (2015). [Google Scholar]
- 118. Chala D., et al. , Good-bye to tropical alpine plant giants under warmer climates? Loss of range and genetic diversity in Lobelia rhynchopetalum . Ecol. Evol. 6 , 8931–8941 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kandziora M., et al. , The enigmatic tropical alpine flora on the African sky islands is young, disturbed, and unsaturated. Proc. Natl. Acad. Sci. 119 , e2112737119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Vihervaara P., et al. , Using long-term ecosystem service and biodiversity data to study the impacts and adaptation options in response to climate change: Insights from the global ILTER sites network. Curr. Opin. Environ. Sustain. 5 , 53–66 (2013). [Google Scholar]
- 121. Hylander K., Frisk C. A., Nemomissa S., Johansson M. U., Rapid post-fire re-assembly of species-rich bryophyte communities in Afroalpine heathlands. Vegetation Science 32 , e13033 (2021). [Google Scholar]
- 122. Mace G. M., The role of taxonomy in species conservation. Philos. Trans. R. Soc. B: Biol. Sci. 359 , 711–719 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fashing N. J., et al. , Bryobia abyssiniae (Prostigmata: Tetranychidae), a new species from the highlands of Ethiopia. Int. J. Acarol. 42 , 366–376 (2016). [Google Scholar]
- 124. Yeshitela K., “Effects of anthropogenic disturbance on the diversity of foliicolous lichens in tropical rainforests of East Africa: Godere (Ethiopia), Budongo (Uganda), and Kakamega (Kenya)“, PhD thesis, Universität Koblenz-Landau; (2008). [Google Scholar]
- 125. Cotterill F. P. D., Systematics, biological knowledge and environmental conservation. Biodivers. Conserv. 4 , 183–205 (1995). [Google Scholar]
- 126. Bergman T. J., Phillips-Conroy J. E., Jolly C. J., Behavioral variation and reproductive success of male baboons (Papio anubis × Papio hamadryas) in a hybrid social group. Am. J. Primatol. 70 , 136–147 (2008). [DOI] [PubMed] [Google Scholar]
- 127. Neil E., Greengrass E., Illegal settlement in the Babile Elephant Sanctuary is threatening the resident elephant population. Oryx 56 , 457–464 (2022). [Google Scholar]
- 128. Johnson P. T. J., de Roode J. C., Fenton A., Why infectious disease research needs community ecology. Science 349 , 6519 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Diagne C., et al. , The economic costs of biological invasions in Africa: A growing but neglected threat? NeoBiota 67 , 11–51 (2021). [Google Scholar]
- 130. Gezie A., et al. , Potential impacts of water hyacinth invasion and management on water quality and human health in Lake Tana watershed, Northwest Ethiopia. Biol. Invasions 20 , 2517–2534 (2018). [Google Scholar]
- 131. Shiferaw H., et al. , Implications of land use/land cover dynamics and Prosopis invasion on ecosystem service values in Afar Region, Ethiopia. Sci. Total Environ. 675 , 354–366 (2019). [DOI] [PubMed] [Google Scholar]
- 132. Sileshi G. W., Gebeyehu S., Mafongoya P. L., The threat of alien invasive insect and mite species to food security in Africa and the need for a continent-wide response. Food Secur. 11 , 763–775 (2019). [Google Scholar]
- 133. Migeon A., Dorkeld F., Spider mites web: A comprehensive database for the Tetranychidae. (2022). https://www1.montpellier.inra.fr/CBGP/spmweb/background.php. Accessed 22 October 2022.
- 134. Assefa F., Ayalew D., Status and control measures of fall armyworm (Spodoptera frugiperda) infestations in maize fields in Ethiopia: A review. Cogent Food Agric. 5 , 1641902 (2019). [Google Scholar]
- 135. Negash M., Yirdaw E., Luukkanen O., Potential of indigenous multistrata agroforests for maintaining native floristic diversity in the south-eastern Rift Valley escarpment, Ethiopia. Agrofor. Syst. 85 , 9–28 (2012). [Google Scholar]
- 136. Funk C., A climate trend analysis of Ethiopia. U.S. Geological Survey fact sheet 2012–3053. (2022). (https://pubs.usgs.gov/fs/2012/3053/FS12-3053_ethiopia.pdf). Accessed 1 July 2016.
- 137. Mountain Research Initiative EDW Working Group, Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 5 , 424 (2015). [Google Scholar]
- 138. Sloan E.T., et al. , Effects of climate variability on the demography of wild geladas. Ecology and Evolution 12 , e8759 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Campos F. A., et al. , Does climate variability influence the demography of wild primates? Evidence from long-term life-history data in seven species. Global Change Biol. 23 , 4907–4921 (2017). [DOI] [PubMed] [Google Scholar]
- 140. Tiedeu B. A., Oluwafunmilayo J.P.-M., Nyambi D., Driving gender equity in African scientific institutions. Lancet 393 , 504–506 (2019). [DOI] [PubMed] [Google Scholar]
- 141. Mekonnen A., et al. , Can I afford to publish? A dilemma for African scholars. Ecol. Lett. 25 , 711–715 (2022). [DOI] [PubMed] [Google Scholar]
- 142. Rochette A.-J., et al. , Developing policy-relevant biodiversity indicators: Lessons learnt from case studies in Africa. Environ. Res. Lett. 14 , 035002 (2019). [Google Scholar]
- 143. Van Noorden R., Five in Asia: Hong Kong, Malaysia, Singapore, South Korea and Taiwan are investing heavily in research as an engine for growth. Nature 558 , 500–501 (2018).29950637 [Google Scholar]
- 144. Laurance W. F., et al. , Averting biodiversity collapse in tropical forest protected areas. Nature 489 , 290 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Data Availability Statement
All data are available in the main text and SI Appendix . Map files for Fig. 1 are available upon request.