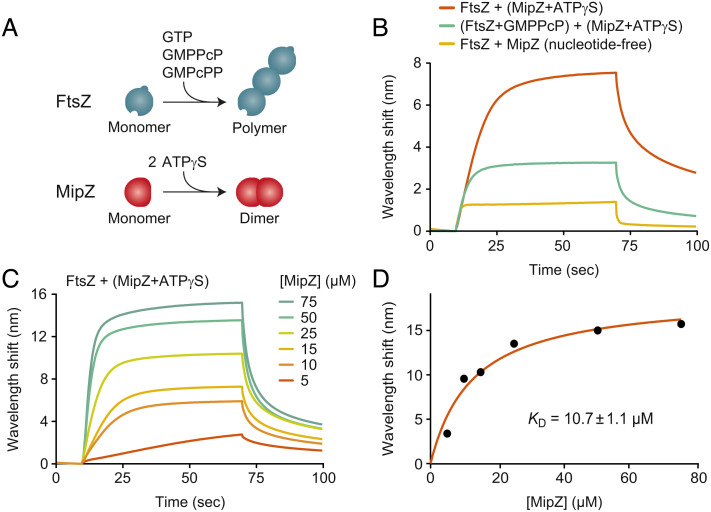
Fig. 1.
MipZ binds directly to FtsZ. (A) Schematics depicting FtsZ polymerization in the presence of GTP or its nonhydrolyzable analogs GMPPcP or GMPcPP (Top) and MipZ dimerization in the presence of ATP or the nonhydrolyzable analog ATPγS (Bottom). (B) Biolayer interferometry analysis of the interaction between FtsZ and MipZ. Biotinylated FtsZ immobilized on a streptavidin-coated biosensor was probed with MipZ (15 µM) in the absence or presence of 1 mM ATPγS or 2 mM GMPPcP. At the end of association phase, the sensor was washed with protein-free buffer to follow MipZ dissociation. Components in parentheses were preincubated together before the start of the binding reaction. Note that 77% of the FtsZ molecules were associated with guanosine-5'-diphosphate (GDP) after purification. (C) Titration series analyzing the interaction of immobilized FtsZ with increasing concentrations (5 to 75 µM) of MipZ in the presence of ATPγS. (D) Binding affinity of MipZ•ATPγS dimers for FtsZ monomers. The wavelength shifts measured at the end of the association phase (Bmax) from panel c were plotted against the protein concentration. The apparent equilibrium dissociation constant (KD) of the FtsZ·MipZ complex was obtained by fitting the data to a one-site saturation ligand binding model. The KD value represents the average of three independent experiments (±SD). The graph shows the results of a representative experiment.