Abstract
Heart failure (HF) and cancer are the leading causes of death worldwide and accumulating evidence demonstrates that HF and cancer affect one another in a bidirectional way. Patients with HF are at increased risk for developing cancer, and HF is associated with accelerated tumour growth. The presence of malignancy may induce systemic metabolic, inflammatory, and microbial alterations resulting in impaired cardiac function. In addition to pathophysiologic mechanisms that are shared between cancer and HF, overlaps also exist between pathways required for normal cardiac physiology and for tumour growth. Therefore, these overlaps may also explain the increased risk for cardiotoxicity and HF as a result of targeted anti-cancer therapies. This review provides an overview of mechanisms involved in the bidirectional connection between HF and cancer, specifically focusing upon current ‘hot-topics’ in these shared mechanisms. It subsequently describes targeted anti-cancer therapies with cardiotoxic potential as a result of overlap between their anti-cancer targets and pathways required for normal cardiac function.
Keywords: Cancer, Heart failure, Cardiotoxic drugs, Mechanisms
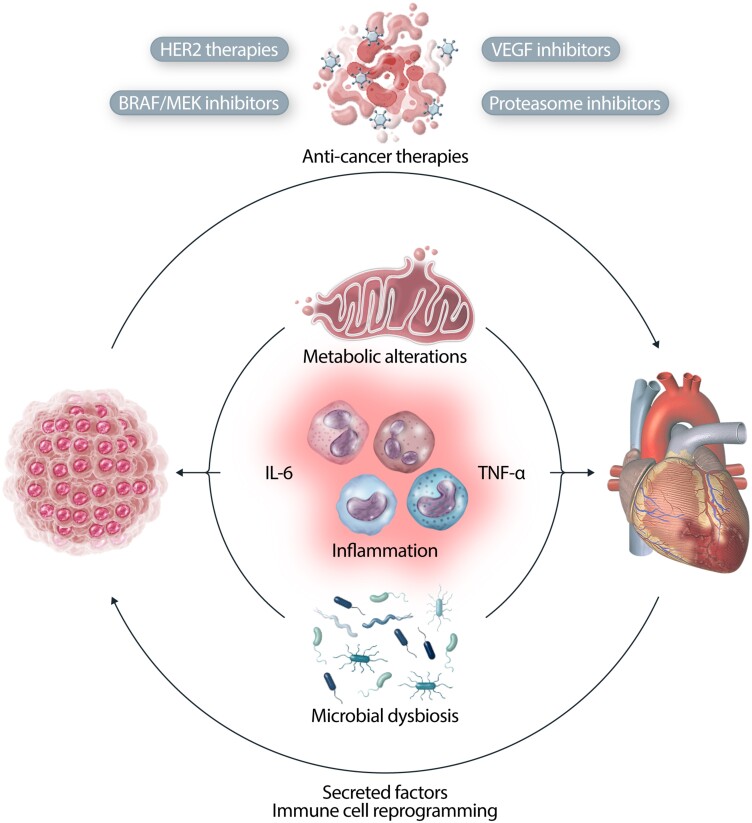
Graphical Abstract
Graphical Abstract.
This article is part of the Spotlight Issue on Heart Failure.
1. Introduction
Cancer is the leading cause of non-cardiovascular (CV) mortality in patients with heart failure (HF),1 accounting for up to 9% of deaths in patients with HF with reduced ejection fraction (HFrEF) and for up to 17% of mortality in patients with HF with preserved ejection fraction (HFpEF).2,3 Conversely, CV disease (CVD) is the most frequent non-cancer cause of death in patients with malignancy.4 In addition to shared risk factors, including obesity, smoking, and diabetes, the mechanistic underpinning of the bidirectional interplay between cancer and HF is becoming clearer.5,6 Cancer and HF both have the potential to provoke profound alterations in cellular homeostasis. These effects are of relevance in isolation, but the substantial overlap between mechanistic pathways of tumour growth and CV physiology may also explain the increased propensity to develop the ‘other’ disease. In addition to pathophysiologic mechanisms that are shared between cancer and HF, overlaps also exist between pathways required for normal cardiac physiology and for tumour growth. Therefore, these overlaps may also explain the increased risk for cardiotoxicity and HF as a result of targeted anti-cancer therapies.
We will provide an overview of the bidirectional interactions between cancer and HF and will describe current ‘hot topic’ mechanisms shared by these conditions. We will also outline the relevance of anti-cancer therapies with potential cardiotoxic effects resulting from overlaps between their anti-cancer targets and pathways required for normal cardiac function.
2. Bidirectional interaction between HF and cancer
2.1. HF as a risk factor for cancer
Over the last decade, a growing number of clinical studies have demonstrated that patients with HF are at increased risk for developing cancer.7–13 The association between HF and cancer was first described in a cohort including 961 patients with HF as well as age- and sex-matched controls. Patients with HF were at increased risk of developing cancer [hazard ratio (HR): 1.68; 95% CI (1.13–2.50)], even after adjustment for body mass index, smoking, and co-morbidities.7 Several more recent studies have corroborated this; the most recent article to be published described a large community based study from the Puglia region of Italy (n = 104 020 subjects), in which cancer incidence and cancer mortality were significantly higher in patients with HF, compared to matched non-HF control subjects [HR: 1.76 (1.71–1.81) and HR: 4.11 (3.86–4.38), respectively].13 These findings were replicated in a German cohort, in which HF was significantly associated with the incidence of cancer [HR: 1.76 (1.71–1.81)].11 In the Women’s Health Initiative study, HF was associated with increased cancer incidence [HR: 1.28 (1.11–1.48)] in female patients. Notably, HFpEF but not HFrEF was associated with increased cancer incidence [HR 1.34, (1.06–1.67); and HR 0.99 (0.74–1.34), respectively].12
The association between HF and cancer can partially be explained by shared risk factors. However, several recent preclinical studies have shown that HF can also stimulate tumour growth directly.14–16 The initial evidence for a causal relation between HF and cancer comes from a study of tumour prone C57BL/6-ApcMin mice, in which tumour growth increased significantly in the context of myocardial infarction (MI)-induced HF.14 To exclude the effect of haemodynamic impairment upon tumour growth, the experiment was repeated in a model of heterotopic heart transplantation. A higher tumour load was observed in mice with a failing heart (whether in situ or transplanted) compared to controls. Several proteins were identified to be increased in the presence of HF and were also associated with proliferative effects in colon cancer cell lines, especially Serpin3A. These observations gave rise to the hypothesis that HF may promote tumour growth through secretion of paracrine factors.14 This hypothesis was recently substantiated in an aortic constriction model, in which early cardiac remodelling, without severe cardiac dysfunction, was seen to promote tumour growth in a breast cancer and lung cancer mouse model.16 Furthermore, plasma obtained from mice subjected to aortic constriction stimulated tumour cellular proliferation, building on the evidence that secreted factors play a role in HF-induced tumour growth. Periostin was identified as a potentially important mediator, given that plasma depleted of periostin no longer evoked tumour proliferative effects.16 Koelwyn et al.15 demonstrated that MI-induced HF increases breast cancer growth via epigenetic remodelling of bone-marrow immune cells which resulted in an immunosuppressed, pro-cancer phenotype. However, despite multiple layers of evidence that HF can stimulate tumour growth, these effects were not reproduced in a mouse model of renal cancer, suggesting that the tumour promoting effects of HF might be cancer-site specific.17
2.2. Cancer as a risk factor for HF
The association between cancer and CVD has been demonstrated in a retrospective cohort study of over 36 000 adults surviving at least two years after a diagnosis of cancer and compared with age and sex matched non-cancer controls. This association varied by cancer type: in comparison with controls, the risk of CVD (including HF) was significantly higher in survivors of multiple myeloma [incidence rate ratio (IRR) 1.7], lung cancer (IRR 1.58) and breast cancer (IRR 1.13).18 However, differentiation of the effect of cancer per se from the cardiotoxic effects of its treatment can be difficult to disentangle in epidemiologic studies. Nonetheless, pre-clinical models show that cancer causes systemic metabolic alterations resulting in impaired cardiac function.19,20 Cachexia represents a systemic manifestation of both cancer and HF.21,22 In animal models, cancer promotes cardiac atrophy and a reduced heart weight with subsequent deterioration in cardiac function.23,24 Cardiac wasting appears to result from increased autophagy and myocyte apoptosis23 with proinflammatory cytokines including tumour necrosis factor-α (TNF-α), interleukin (IL) 1β, and IL-6 playing pathophysiological roles.24
3. Mechanistic overlap in cancer and HF pathophysiology
3.1. Metabolic alterations
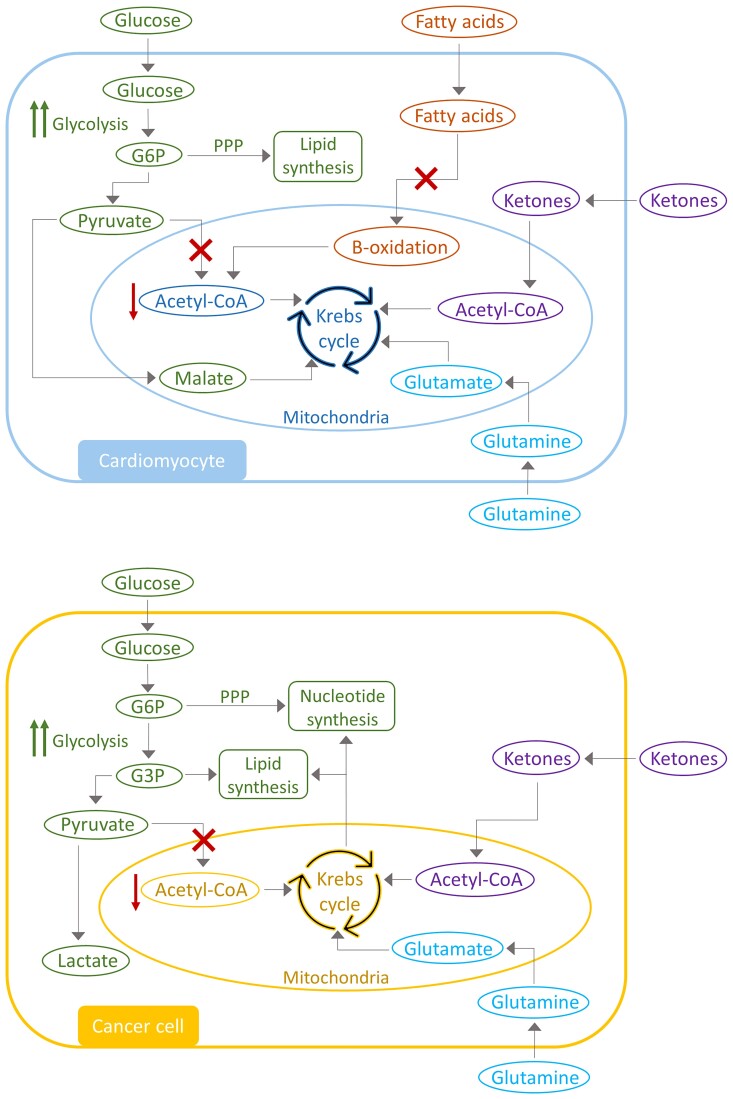
Metabolic remodelling is considered a hallmark in the pathophysiology of both cancer and HF, and has been the focus for new treatment strategies for both diseases in recent decades.25,26 HF and cancer are characterized by several common metabolic alterations (Figure 1), which begs the question as to whether metabolic derailment might play a role in the connection between HF and cancer. It remains largely unknown if metabolic switches in cancer, either in the tumour or surrounding tissues, affect the CV system. Vice versa, metabolic changes in the heart are unlikely to cause cancer development, but well-described metabolic repercussions of CVD, such as insulin insensitivity and diabetes,28 are clearly associated with an increased risk for cancer.
Figure 1.
Metabolic alterations in heart failure and cancer. Heart failure and cancer are characterized by several overlapping changes in metabolic pathways. Both diseases are characterized by an increase in glycolysis and a decrease in oxidative respiration. Glycolytic intermediates are redirected into branch pathways for nucleotide and lipid synthesis. Alternative fuel sources are used for the Krebs cycle, a mechanism called anaplerosis, to compensate for the decreased acetyl-CoA levels for ATP production. Adjusted from DeBerardinis et al.26 and Garcia-Ropero et al.27
3.1.1. Switch to glycolysis
One of the major commonalities in metabolic remodelling between HF and cancer is the shift in metabolic dependency, favouring glycolysis over oxidative phosphorylation. In the healthy heart, the majority of adenosine triphosphate (ATP) is produced through fatty acid (FA) oxidation and only small amounts through oxidation of glucose and ketone bodies.29 In the failing heart, however, glycolytic activity is increased,30,31 while FA and glucose oxidation is substantially decreased.30,32 The consequence of this is a net decrease in acetyl-coenzyme A (CoA) bioavailability for ATP production via the Krebs cycle. Similarly, cancer metabolism is also characterized by an increase in glycolytic activity. As long ago as 1927, Otto Warburg33 provided evidence that cancer cells obtain glucose and produce lactate irrespective of oxygen availability.34 Several oncogenes and tumour-suppressor genes are involved in the increased glycolysis. Specifically, phosphoinositide 3-kinases (PI3K), Ak transforming factor (Akt) and MYC proto-oncogene (MYC) are known to upregulate transcription and translocation of Glucose transporter 1, and increase hexokinase activity.35,36 P53, possibly the most well-known tumour suppressor gene in the field of oncology, has also been associated with metabolic remodelling, since a loss of P53 results in increased glycolytic flux.37
3.1.2. Alternative fuel and anaplerosis
Although both cancer and HF are characterized by an increase in glycolysis, glucose is not believed to be the major energy source in either disease. In end-stage HF, ATP levels only decrease by 60–70% from normal capacity.38,39 In the oncology field, several studies have shown that mitochondrial metabolism is crucial for tumour proliferation40,41 and experimental inhibition of glycolytic ATP production, via the inhibition of pyruvate kinase, does not result in reduced tumourigenesis.42 These findings suggest that compensatory mechanisms are activated in HF and cancer to maintain mitochondrial ATP production.
Anaplerosis represents a mechanism via which the Krebs cycle is fuelled by intermediaries independent of acetyl-CoA. This mechanism plays an important role in both HF and cancer metabolism. Indeed, anaplerotic flux is increased in the context of cardiac hypertrophy43,44 and also in cancer.45,46 In a pressure-overload induced HF model, pyruvate was converted to malate, which can enter the Krebs cycle.43 Additionally, glutamine utilization is increased in HF and cancer.47,48 Its subsequent conversion to glutamate is followed by the production of α-ketoglutarate, an intermediate for the Krebs cycle and, even in hypoxic conditions, glutamine is utilized for oxidative ATP production in cancer cells.49,50 Ketone bodies are utilized as an alternative energy source in HF and this phenomenon has been reported in patients with HF51 as well as animal models of HF.52 This is believed to be an important adaptive mechanism in the setting of decreased FA oxidation. The role of ketones in cancer metabolism is less well understood but several studies have provided evidence that ketone body utilization can stimulate tumour growth,53,54 although data are conflicting and treatment with ketone bodies has also been reported to decrease tumour growth.55,56
In both cancer and HF, glycolytic intermediates are often redirected to a branching pathway for biosynthesis, instead of being directed to the Krebs cycle. In cancer, glucose-6-phosphate (G6P) is redirected to the pentose phosphate pathway (PPP) for nucleotide biosynthesis and glyceraldehyde-3-phosphate can be converted to glycerol-3-phosphate for lipid synthesis.57,58 In addition, Krebs intermediates are used to produce cytosolic aspartate and acetyl-CoA for nucleotide and lipid synthesis.59,60 In HF, G6P is also redirected to the PPP for production of nicotinamide adenine dinucleotide phosphate (NADPH), which is essential for regulating oxidative stress and lipid synthesis.31 These diversions of glycolytic intermediates have a negative effect upon cardiac energetics and function. Furthermore, G6P may also enter the hexosamine biosynthetic pathway, leading to increased O-GlcNAcylation, which is further associated with HF.61,62
3.1.3. Hypoxia
Hypoxia induced factor-1 (HIF-1) is a key regulator of metabolic adaptation in cancer and HF. Proliferation of tumour cells often exceeds angiogenesis, resulting in a hypoxic environment and HIF-1 activation.26 Sustained activation of HIF-1 is also induced in cancer under normoxic conditions due to mutations in the mammalian target of rapamycin complex 1 (mTORC1) pathway or von Hippel-Lindau (VHL).63 HIF-1 regulates transcription of proteins involved in glucose metabolism and protein, lipid and nucleotide biosynthesis64 and has been shown to promote tumour growth.65–67 Interestingly, patients with VHL syndrome, characterized by sustained HIF-1 activation and development of tumours in numerous organs,68 also develop cardiopulmonary abnormalities.69,70 VHL knockout mice develop cardiac lipid accumulation, fibrosis and apoptosis.71 Increased HIF-1 expression also induces cardiac hypertrophy by regulating enzymes involved in FA and glucose metabolism.72–74 HIF-1 levels are higher in patients with hypertrophic cardiomyopathy than they are in healthy people and transverse aortic constriction (TAC) is associated with increased HIF1. Notably, ventricular depletion of HIF1α prevents TAC-induced cardiac dysfunction.72
3.1.4. Metabolic targets in cancer and HF therapy
Because of its central role in both diseases, metabolic derangement may be a valid target in cardio-oncology. Indeed, treatments targeting metabolism in HF and cancer have been studied extensively. One promising treatment might be with sodium-glucose co-transporter 2 inhibitors (SGLT2i) which were originally developed as anti-diabetic drugs.75 SGLT2i drugs reduce CV events and reduce worsening HF in both diabetic and non-diabetic HF patients.76,77 Several preclinical studies have shown that treatment with SGLT2i can positively alter cardiac metabolism in HF models, resulting in reduced cardiac remodelling.78–80 The effect of SGLT2i on tumour growth has also been studied. Both in vitro and in vivo, treatment with SGLT2i inhibited tumour growth of several obesity and diabetes-associated cancer models.81–83 Furthermore, the SGLT2i, canagliflozin, inhibits tumour growth even in tumour models without obesity or diabetes, potentially via its effects on cellular glucose levels.84,85
Many studies have focused on targeting HIF-1 as an anti-cancer treatment and this has been extensively reviewed elsewhere.86 Indeed, HIF-1 inhibition has shown promising anti-tumourigenic effects in renal,87,88 hepatocellular,89 and breast cancers.90 Downregulation of HIF1 in a mouse model ischaemia/reperfusion attenuated cardiac injury after reperfusion.91 Interestingly, treatment with Belzutifan, an inhibitor of the HIF2 isoform, has shown promising anti-tumourigenic effects in renal cancer, was shown to attenuate pulmonary hypertension and fibrosis in mice with a VHL mutation.92
3.2. Inflammation
Inflammation has frequently been considered as a nodal point linking HF and cancer. Heart disease and cancer are associated with an increase in pro-inflammatory cytokines, including TNF-α and IL-1β93–96 and chronic inflammation increases the risk of new onset cancer97,98 and CVD.99 After MI, the innate immune system is activated leading to a pro-inflammatory response, which is initially cardio-protective. However, prolonged activation of pro-inflammatory signalling, especially via IL-6, induces cardiac remodelling and cardiac dysfunction.100,101 Importantly, pro-inflammatory cytokines also promote tumourigenesis.102,103 In addition, the tumour-microenvironment is infiltrated by tumour associated macrophages, which produce cytokines to stimulate angiogenesis and inhibit the anti-tumour response of cytotoxic T-cells.104,105
3.2.1. HF-associated inflammation
Patients with HF and elevated C-reactive protein (CRP) (>2 mg/L) have an increased risk of cancer.14,106,107 In a pre-clinical model of MI-induced HF described previously, the subsequent effect of HF upon increased tumour growth was accompanied by elevated circulating concentrations of pro-cancer chemokines, such as chemokine (C-X-C motif) ligand (CXCL13). In these animals with HF, there was also an increase in monocytic myeloid-derived suppressor cells found in the tumour tissue and these suppressed CD8 + cytotoxic T cell activity, further potentiating tumour growth.15
3.2.2. Clonal haematopoiesis of indeterminate potential-associated inflammation
Clonal haematopoiesis of indeterminate potential (CHIP) reflects the accumulation of somatic, potentially pro-leukemic mutations in haematopoietic stem cells, occurring in the absence of haematological malignancy.108 The commonest mutations found in CHIP occur in genes that are also important in the regulation of inflammation.109 These include mutations occurring in the driver genes DNA methyltransferase 3A (DNMT3A), ten-eleven-translocation-2 (TET2), Janus kinase 2 (Jak2) and additional sex comb-like 1 (ASXL1). Although the risk of malignant transformation is low (<1% per year), CHIP carriers have an excess risk of mortality which reflects a heightened risk for CV events including MI and stroke,109–112 as well as an association with increased HF hospitalization and HF mortality.113,114 In a large study including five population-based cohorts and over 50 000 participants, CHIP correlated with a 25% increased risk for new onset HF.115 Clonal haematopoiesis initiates a pro-inflammatory state associated with high circulating levels of pro-inflammatory markers in humans.116,117 Pre-clinical studies demonstrated that haematopoietic mutations in TET2, DMNT3A, and Jak2 lead to an accelerated HF phenotype in several mouse HF models, accompanied by an increase in pro-inflammatory cytokines, including IL-1β and IL-6.118–120
3.2.3. Obesity-associated inflammation
Obesity is characterized by chronic inflammation. In 2016, 39% of the adult world population was overweight, of whom 14% were obese.121 In the lean state, adipose tissue is infiltrated by anti-inflammatory immune cells which are important regulators of insulin sensitivity.122,123 However, in the obese state, a shift in constituent immune cells occurs with a relative decrease in anti-inflammatory components and an increase in pro-inflammatory Th1 and CD8+ T cells. In addition, a shift occurs in the macrophage phenotype, with increased M1-like macrophages.124 This is accompanied by an increase in pro-inflammatory cytokines, chemokines, and adipokines, such as leptin. Numerous studies have shown that obesity increases the risk of a wide range of malignancies, including breast, colorectal, and liver cancer.125–129 Cytokines and adipokines secreted by adipose tissue stimulate tumour growth and progression, including IL-6, TNFα, and leptin.130,131 In addition, cancer-associated adipocytes may also be present in the tumour-microenvironment and can further stimulate tumour progression.132 Obesity is also associated with and increased risk of HF, especially HFpEF133–136 and it is notable that leptin-resistant db/db mice develop a HFpEF phenotype, with evidence of cardiac hypertrophy and interstitial fibrosis.137 IL-6, TNFα, and leptin also induce hypertension and atherosclerosis,138,139 both of which represent major pathogenetic processes in the development of HF.
3.2.4. Inflammation as a therapeutic target in HF and cancer
Considering the role of inflammation in both diseases, targeting inflammatory mechanisms in HF and cancer could have therapeutic potential. Indeed, commonly used HF medications, such as statins, have some anti-inflammatory properties.140 In the Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS), treatment with canakinumab, a monoclonal IL-1β antibody, reduced the rate of recurrent atherosclerotic CV events in patients with previous MI and high CRP levels (<2 mg/L).141 Notably, canakinumab also reduced HF hospitalization and HF-related mortality by 23% in patients who achieved a CRP level of <2 mg/L.142 A sub-analysis of the CANTOS study showed that treatment with Canakinumab also decreased the incidence of lung cancer143 and is being investigated further as a treatment for that indication.144
Targeting inflammatory chemokines as a therapeutic strategy for HF and cancer has also generated interest. Chemokines including Chemokine (C-C Motif) Ligand 2 (CCL2) and CXCL13 play a pivotal role in cancer145,146 and circulating levels are also increased in patients147,148 and preclinical models of HF.149 Preclinical studies using CCL2 or CXCL13 inhibitors have reported treatment-related reductions in tumour proliferation.150–153 CCL2 knockout attenuates cardiac remodelling after ischaemia/reperfusion injury, but CCL2 knockout was also associated with delayed replacement of injured cardiomyocytes with connective tissue, a process that is essential after infarction.149 Initial clinical trials examining the use of chemokine inhibitors in cancer have shown promise.154 However, clinical studies in patients with HF have not yielded positive results so far.155 A better understanding of the complex role of chemokines in the pathophysiology of cancer and HF is required in order to maximize the potential of this potential strategy.
The complex inter-relationship between the immune system, cancer and HF is exemplified by immune checkpoint inhibitors in use as anti-cancer therapy. These potent anti-cancer drugs now have a very broad, and growing, range of indications in oncology and are associated with remarkable cancer outcomes.156,157 By inhibiting immune checkpoints on cancers cells, a T cell mediated immune response is initiated, allowing immune targeting of cancer cells.156 However, myocarditis occurs in up to 2% of patients treated with these agents and CV mortality associated with these events can be up to 40%.158–160
3.3. Microbiome
The human microbiome is comprised of trillions of bacteria, archaea, and eukaryotic microbes, which maintain a mutualistic relationship with their host.161,162 Gut microbiota are essential for fermentation of dietary fibres and vitamin biosynthesis, and play an important role in intestinal health and immune regulation.161,163 The microbial composition is greatly affected by environmental factors such as food and dietary patterns, smoking and drug use. These factors can influence the microbial diversity and the abundance of specific microbial species, resulting in microbial dysbiosis.164 People with obesity, for instance, show decreased microbial diversity.165 Accumulating evidence is emerging on the bidirectional connection between the microbiome, HF and cancer. HF and cancer, and their therapies, are believed to affect the microbial composition in several ways and microbial dysbiosis can play a role in both diseases as outlined below and in Figure 2.
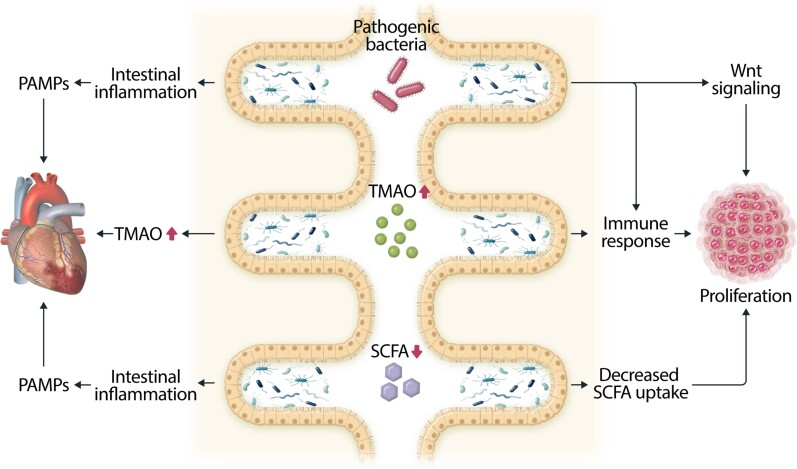
Figure 2.
The overlapping effects of microbial dysbiosis in HF and cancer. Overgrowth of pathogenic bacteria and decreased SCFA levels can induce intestinal inflammation, resulting in increased circulating PAMPs, which can induce cardiac fibrosis and dysfunction. Increased circulating levels of microbial metabolite TMAO can induce atherosclerotic plaque formation and HF. In cancer increased TMAO and pathogenic bacteria can directly promote tumour growth by activating immune cells. In addition, pathogenic bacteria can directly promote tumourigenesis by activating Wnt/β-catenin signalling. Decreased SCFA levels are favourable for tumourigenesis and tumour cells actively inhibit SCFA uptake.
3.3.1. Microbial dysbiosis in cancer and HF
The role of the gut microbiome in colorectal cancer (CRC) has been studied extensively. Patients with CRC exhibit a distinct microbial composition, characterized by an increase in Fusobacteria and pathogenic bacteria and a decrease in Clostridiales, Faecalibacterium, and Bifidobacterium.166–168 In addition, microbial dysbiosis has been associated with an increased incidence of CRC.166,167 In vivo studies have provided direct evidence that microbiota from patients with CRC can promote tumourigenesis.169,170 In these studies, stool samples from patients with CRC were transplanted into a colon cancer mouse model, or germ-free mice. Stool samples from patients had different microbial composition than stool samples from healthy individuals, and transplantation of microbiota from CRC patients led to increased tumour growth in the colon cancer model and increased colonocyte proliferation in the germ-free mice.169,170 Microbial dysbiosis has also been associated with cancer in other organs, including breast and liver.171,172
Substantial clinical evidence now exists to show that patients with HF also have a distinct gut microbial composition.173–175 The microbiome and HF appear to affect one another in a bidirectional way. Differences in microbial composition can increase atherosclerotic plaque formation and can lead to a worsening HF phenotype in mouse models.176–178 On the other hand, HF may cause gut congestion and low-grade inflammation, resulting in increased intestinal permeability, and consequent microbial dysbiosis.179,180 In addition, HF medication plays a role in the equation. HF medication can strongly affect the microbial composition 181,182 and vice versa, the microbiome can affect the response on HF drugs, altering the effectiveness of treatment.183
Notably, there are several similarities in between microbial patterns observed in cancer and those observed in HF, including an increase in Fusobacteria and a decrease in Clostridiales, and Bifidobacterium. Furthermore, the microbiome has been shown to influence both HF and cancer via mechanisms including pathogenic bacteria, microbial metabolites and short chain FAs (SCFAs) (Figure 2).
3.2.2. Pathogenic bacteria
Several bacteria have been identified to have direct tumourigenic effects, including Helicobacter pylori, Fusobacterium nucleatum, Bacteroides fragilis, and Salmonella enterica. H. pylori bind to intestinal epithelial cells and interact with Wnt/β-catenin signalling, directly regulating cell proliferation, inflammation and apoptosis.184 F. nucleatum has the ability to induce tumour proliferation by inducing a proinflammatory response through activation of the nuclear factor-κB (NF-κB) pathway185 and is associated with epithelial-to mesenchymal transition.186 B. fragilis targets intestinal tight junctions through activation of the Wnt/β-catenin and Nf-kB pathways, resulting in barrier disruption and intestinal inflammation187 and can induce proliferation through activation of celullar MYC (C-MYC).188,189
Several pathogenic bacteria are more abundant in patients with HF. Pathogenic bacteria affect gut barrier integrity, resulting in bacterial translocation and chronic inflammation, which has been associated with CVD.190 Indeed, systemic levels of several pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), are increased in patients with HF.191 LPS exposure leads to cardiac fibrosis and dysfunction in mice.192,193
3.3.3. Trimethylamine-N-oxide
Arguably the best characterized link between HF and the microbiome is that of trimethylamine-N-oxide (TMAO).194,195 This microbial metabolite is generated from nutrients containing trimethylamine (TMA), including choline and l-carnitine. TMA is transported to the liver where it is further metabolized to TMAO. Circulating TMAO levels are associated with an increased risk for CVD and HF, and worse outcomes.196–199 These observations have been corroborated in preclinical studies, which provide evidence of direct effects of TMAO on atherosclerotic plaque formation and HF.176–178
There is increasing evidence to suggest that TMAO also plays a role in tumour growth. Higher TMAO levels have been associated with an increased risk for CRC in several studies.200–202 However, not all investigators have demonstrated this link in CRC.203 It is of note that any association between and TMAO and cancer may extend beyond the gastrointestinal tract and higher TMAO levels have also been associated with prostate cancer and oral squamous cell carcinoma.204,205 It is currently hypothesized that TMAO can induce tumourigenic effects by activating a chronic immune response.206,207
3.3.4. SCFAs
A key signature in the microbial composition found in patients with CRC as well as patients with HF is the substantially lower number of SCFA-producing bacteria, such as Roseburia and Lachnospiraceae.168,175,208,209 SCFAs, including acetate, propionate and butyrate, are fermentation products of dietary fibres, produced by gut microbiota. Butyrate is the main energy source for colonocytes and is essential for maintaining colonic health, mucus production, barrier integrity and immune regulation.210 However, cancer cells switch energy source, favouring glucose over butyrate,211 and butyrate uptake in colon cancer cells is substantially decreased.212,213 Furthermore, several preclinical studies provide evidence that butyrate possesses anti-tumourigenic properties and that butyrate treatment inhibits tumour growth in CRC cell lines.214,215
Low SCFA levels are also associated with hypertension and HF. Decreased SCFA levels can cause intestinal barrier dysfunction, resulting in translocation of microbial metabolites as well as inflammation.216 Plasma SCFA levels are decreased in patients with HF.217 and SCFA supplementation attenuates HF in mice.218–220 Butyrate supplementation protected from doxorubicin induced cardiotoxicity in vivo.218 Propionate induces vasodilatation and antibiotic-induced depletion of propionate is associated with increased blood pressure in mice.221 Whether or not this afterload effect is a relevant mechanism for the development or worsening of HF in humans is unknown.
4. Mechanistic overlap in anti-cancer targeted therapies and cardiac physiologic pathways
In addition to shared pathophysiological mechanisms in cancer and HF, there are also substantial overlaps between pathways required for tumour growth and those required for normal cardiac function. These overlapping effects make substantial contributions to the potential cardiotoxic effects of a growing number of anti-cancer therapies, with consequent left ventricular dysfunction and HF. In contrast to traditional chemotherapeutic agents, the majority of novel anti-cancer therapies are targeted and act upon specific cancer signalling pathways. Numerous targeted therapies have been associated with HF, including anti-human epidermal growth factor receptor 2 (HER2) therapies, vascular endothelial growth factor (VEGF) signalling pathway inhibitors, rapidly accelerate fibrosarcoma B-type (BRAF), and mitogen-activated extracellular signal-regulated kinase (MEK) inhibitors and proteasome inhibitors (Table 1).
Table 1.
Mechanisms of cardiotoxicity due to targeted therapies
| Targeted therapy class | Examples | Overlapping signalling pathways for cancer growth and cardiac physiology | Proposed mechanisms of cardiotoxicity |
|---|---|---|---|
| HER2 + targeted therapy | Trastuzumab Pertuzumab | HER2 NRG-1 HER4 PI3K/Akt MEK/ERK Src/FAK mTOR | Impaired cell proliferation |
| Impaired angiogenesis | |||
| Impaired cardiomyocyte metabolism | |||
| Impaired autophagy | |||
| Impaired mitochondrial function | |||
| VEGF inhibitors | Axitinib Cabozantinib Lenvatinib Pazopanib Sunitinib Sorafenib Vandetanib | RAF/MEK/ERK PI3K/Akt VEGFR PDGFR c-Kit AMPK | Hypertension |
| Capillary rarefaction | |||
| Cardiomyocyte apoptosis | |||
| Oxidative stress | |||
| BRAF/MEK inhibitors | Dabrafenib + Trametinib Encorafenib + Binimetinib Vemurafenib + Cobimetinib | RAF/MEK/ERK | Hypertension |
| Cardiomyocyte apoptosis | |||
| Impaired cardiomyocyte hypertrophy | |||
| Proteasome inhibitors | Bortezomib Carfilzomib Ixazomib | Ubiquitin-proteasome system | Altered protein homeostasis |
| Accumulation of misfolded proteins | |||
| Endothelial dysfunction | |||
| Cardiomyocyte apoptosis |
HER4, human epidermal growth factor receptor 4; RAF, rapidly accelerate fibrosarcoma.
4.1. Anti-HER2-targeted therapies
The transmembrane tyrosine kinase receptor HER2 is overexpressed in approximately 20% of breast cancers.222 Trastuzumab is a monoclonal antibody against HER2 and improves survival in patients with HER2 positive breast cancer. However, it is strongly associated with cardiac toxicity. A meta-analysis of ten randomized controlled trials of trastuzumab reported that, during trial follow-up, asymptomatic left ventricular systolic dysfunction (LVSD) and symptomatic HF occurred in 7.5 and 1.9% respectively.223 Registry data from the United States of America reveal that the incidence of HF is up to 6% at 1 year and 20% at 5 years.224
HER2 and its ligand, neuregulin-1 (NRG-1), are essential for embryonic heart development, cardiomyocyte growth and survival and maintaining cardiac function in the adult heart.225,226 Cardiac-specific HER2 murine knock-out animals are apparently normal at birth but develop dilated cardiomyopathy as they age.226,227 In the physiologic state, homo- or hetero-dimerization of HER2 activates downstream signalling pathways including the PI3K/Akt which protects cardiomyocytes from apoptosis and also activates MEK/extracellular signal-regulated kinase (ERK) pathways which promote cardiomyocyte growth and proliferation.228
Trastuzumab binds to the extracellular segment of HER2 receptors and blocks downstream PI3K/Akt activity while pertuzumab, a more recently introduced anti-HER2 monoclonal antibody, inhibits signalling via inhibition of dimerization.229 Inhibition of HER2 results in a downregulation of endothelial nitric oxide synthase (eNOS) expression, accumulation of reactive oxygen species (ROS) and subsequent acceleration of apoptosis causing oxidative stress and cell injury.230 Furthermore, inhibition of HER2 downregulates the MEK/ERK and Src/focal adhesion kinase (FAK) pathways resulting in disordered myocardial structure.231 Recent studies have also shown that inhibition of HER2 with trastuzumab, in human primary cardiomyocytes, activates the Erk/mechanistic target of rapamycin (mTOR) cascade which leads to autophagy inhibition, ROS accumulation and reduced mitochondrial function.232
4.2. VEGF signalling pathway inhibitors
Angiogenesis, the process of new blood vessel growth, is vital for the nutrient supply and growth of solid organ cancers. VEGF is the most potent angiogenic factor and VEGF signalling pathway inhibitors (VSPIs) are effective anti-cancer therapies used in the treatment of a wide range of cancers including renal, hepatocellular and thyroid cancers, gastrointestinal stromal tumours, sarcoma and others. Numerous VSPIs have been developed including monoclonal antibodies and tyrosine kinase inhibitors (TKIs). Hypertension is the most commonly described CV adverse effect associated with VSPIs233,234 but these drugs are also associated with LVSD and HF.235 Meta-analysis of 21 trials including several VSPIs reported an incidence of LVSD of 2.4%.235 In addition to a fundamental role in the control of angiogenesis, VEGF signalling plays a pivotal role in endothelial cell proliferation and survival236 and acts as a compensatory mechanism in response to cardiac stressors. VEGF is secreted in response to hypertension and ischaemia and plays a key role in cardiomyocyte hypertrophy.237,238
Mechanisms of cardiotoxicity and HF related to VEGF inhibitors are thought to result from a combination of direct myocardial toxicity due to a reduction of cardioprotective signalling and increased cardiac afterload. The inhibition of VEGF signalling in animal models of pressure overload leads to capillary rarefaction, reduced contractile function and the development of HF.239 Preliminary data suggest a role for cardiac microvascular dysfunction in the development of LVSD in patients treated with VSPI.240 Additionally, VEGF TKIs also act on a range of non-VEGF targets and this varies between drugs. While sometimes intended to increase anti-cancer effects, these non-VEGF target effects may also be unintended or incompletely defined. Potentially cardiotoxic non-VEGF or ‘off-target’ effects include inhibition of platelet derived growth factor receptor (PDGFR) or adenosine monophosphate kinase (AMPK) downregulation.241,242
4.3. BRAF and MEK inhibitors
BRAF and MEK are key components of the mitogen activated protein kinase (MAPK) pathway, a key regulator of normal cell growth and proliferation. Mutation of the BRAF gene results in constitutive activation of BRAF’s kinase function and may be found in patients with melanoma,243 non-small cell lung cancer244 and CRC.245 The use of these drugs has had a profound impact on outcomes for patients with melanoma in particular. Inhibition of BRAF alone is associated with drug-resistance due to paradoxical hyperactivation of MEK, and combined inhibition of BRAF and MEK helps minimize resistance and improve outcomes.246 Treatment with BRAF and MEK inhibitors in combination is associated with an increased risk of HF compared to BRAF inhibitor monotherapy. Meta-analysis of five randomized controlled trials reported reduction in LVEF in 8.1% of patients in the combination therapy group compared to 2% in the BRAF inhibitor monotherapy group.247
Activation of MAPK signalling leads to a cascade of phosphorylation events and ultimately the activation of ERK. Activated ERK provokes the phosphorylation of several targets involved in the regulation of key cellular activities.248 As such, the MAPK pathway is a key component in processes including myocyte hypertrophy, cardiac remodelling and myocardial cell death.249 Animal models have also demonstrated that the MEK/ERK signalling pathway is required for the protection of myocardium following ischaemic injury.250 Disruption of the MAPK pathway by BRAF and MEK inhibitors could therefore lead to a change in these physiological cardioprotective mechanisms and affect apoptosis, remodelling and hypertrophy, ultimately leading to LVSD and HF.249,251 In mouse models, ERK null mice have normal cardiac function but are more susceptible to a subsequent cardiac insult.250 Therefore, a ‘second hit’ such as hypertension or ischaemia be the trigger to LVSD in the context of BRAF and MEK inhibitor exposure.252
4.4. Proteasome inhibitors
The proteasome is a protein complex which plays an important role in intracellular protein degradation and influences a number of intracellular processes.253 Proteasome inhibition leads to an accumulation of misfolded intracellular proteins, an unfolded protein stress response, with subsequent cell-cycle arrest and apoptosis which is toxic to cancer cells. Proteasome inhibitors including bortezomib, carfilzomib and ixazomib are used in the treatment of haematological malignancies including multiple myeloma and certain lymphomas.254 Meta-analysis of 25 clinical trials showed that bortezomib did not significantly increase the risk of cardiotoxicity compared to control patients.255 However, meta-analysis of 24 clinical trials of carfilzomib reported an incidence of HF of 4.1%256 which may be a reflection of carfilzomib’s irreversible action.
The ubiquitin-proteasome system is essential for the turnover of damaged or misfolded proteins to balance the synthesis of new proteins in the heart.257 Specific proteins are labelled with ubiquitin molecules identifying them for degradation by the proteasome. Optimal cardiomyocyte function is dependent on this equilibrium between protein synthesis and turnover. Patients with advanced HF and hypertrophic cardiomyopathy have reduced myocardial proteasome activity with a resulting relative increase in the ratio between protein synthesis and degradation.258 Bortezomib reversibly inhibits and carfilzomib irreversibly inhibits the 26S proteasome.254 In vitro studies have shown that both bortezomib and carfilzomib are directly toxic to cardiomyocytes and induce apoptosis.259 Mouse models with genetically modified ubiquitin-proteasome activity and pressure overload showed a marked increase in cardiomyocyte death causing rapidly progressive HF.260 A recent study demonstrated that dysregulation of the ubiquitin-proteasome system and expression of truncated titin proteins is implicated in the pathogenesis of dilated cardiomyopathy associated with truncating variants in the TTN gene.261 Engineered muscle generated from human induced pluripotent stem cell-derived cardiomyocytes with truncating variants in TTN showed an improvement in function in response to proteasome inhibition.261 This is in contrast to the effects of proteasome inhibition seen in the context of cancer therapy and further work is welcomed to enhance knowledge in this area.
5. Conclusion
The bidirectional interplay between cancer and HF is substantial and relates to several fundamental mechanisms. It now remains to be seen whether therapeutic targeting of these common pathophysiologic pathways can be harnessed to allow improved outcomes for patients affected by HF or cancer or, indeed, in the growing population affected by both. Furthermore, the adverse cardiac effects of a growing number of targeted anti-cancer therapies have, inadvertently, provided insight into the relevance of pathways required for normal cardiac function. These overlaps serve to further reinforce the growing relevance and need for close collaboration between cancer and CV specialists, in clinical, basic science, and drug development settings.
Contributor Information
Sanne de Wit, Department of Cardiology, University Medical Centre Groningen, University of Groningen, PO Box 30.001, Hanzeplein 1, 9700 RB, Groningen, The Netherlands.
Claire Glen, Institute of Cardiovascular and Medical Sciences, University of Glasgow, 126 University Place, Glasgow G12 8TA, United Kingdom.
Rudolf A de Boer, Department of Cardiology, University Medical Centre Groningen, University of Groningen, PO Box 30.001, Hanzeplein 1, 9700 RB, Groningen, The Netherlands.
Ninian N Lang, Institute of Cardiovascular and Medical Sciences, University of Glasgow, 126 University Place, Glasgow G12 8TA, United Kingdom.
Funding
N.N.L. is supported by a British Heart Foundation Centre of Research Excellence Grant (RE/18/6/34217). R.A.d.B. is supported by the European Research Council (ERC CoG 818715), by the Netherlands Heart Foundation (grants 2017–21; 2017–11; 2018–30; 2020B005), and by the Fondation LeDucq (Cure-PLaN).
References
- 1. Moliner P, Lupón J, de Antonio M, Domingo M, Santiago-Vacas E, Zamora E, Cediel G, Santesmases J, Díez-Quevedo C, Troya MI, Boldó M, Altmir S, Alonso N, González B, Núñez J, Bayes-Genis A. Trends in modes of death in heart failure over the last two decades: less sudden death but cancer deaths on the rise. Eur J Heart Fail 2019;21:1259–1266. [DOI] [PubMed] [Google Scholar]
- 2. Boer RA, Meijers WC, Meer P, Veldhuisen DJ. Cancer and heart disease: associations and relations. Eur J Heart Fail 2019;21:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vergaro G, Ghionzoli N, Innocenti L, Taddei C, Giannoni A, Valleggi A, Borrelli C, Senni M, Passino C, Emdin M. Noncardiac versus cardiac mortality in heart failure with preserved, midrange, and reduced ejection fraction. J Am Heart Assoc 2019;8:e013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Boer RA, Hulot JS, Tocchetti CG, Aboumsallem JP, Ameri P, Anker SD, Bauersachs J, Bertero E, Coats AJS, Čelutkienė J, Chioncel O, Dodion P, Eschenhagen T, Farmakis D, Bayes-Genis A, Jäger D, Jankowska EA, Kitsis RN, Konety SH, Larkin J, Lehmann L, Lenihan DJ, Maack C, Moslehi JJ, Müller OJ, Nowak-Sliwinska P, Piepoli MF, Ponikowski P, Pudil R, Rainer PP, Ruschitzka F, Sawyer D, Seferovic PM, Suter T, Thum T, van der Meer P, Van Laake LW, von Haehling S, Heymans S, Lyon AR, Backs J. Common mechanistic pathways in cancer and heart failure. A scientific roadmap on behalf of the translational research committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertero E, Canepa M, Maack C, Ameri P. Linking heart failure to cancer background evidence and research perspectives. Circulation 2018;138:735–742. [DOI] [PubMed] [Google Scholar]
- 6. Tocchetti CG, Ameri P, de Boer RA, D’Alessandra Y, Russo M, Sorriento D, Ciccarelli M, Kiss B, Bertrand L, Dawson D, Falcao-Pires I, Giacca M, Hamdani N, Linke WA, Mayr M, van der Velden J, Zacchigna S, Ghigo A, Hirsch E, Lyon AR, Görbe A, Ferdinandy P, Madonna R, Heymans S, Thum T. Cardiac dysfunction in cancer patients: beyond direct cardiomyocyte damage of anticancer drugs: novel cardio-oncology insights from the joint 2019 meeting of the ESC working groups of myocardial function and cellular biology of the heart. Cardiovasc Res 2020;116:1820–1834. [DOI] [PubMed] [Google Scholar]
- 7. Hasin T, Gerber Y, McNallan SM, Weston SA, Kushwaha SS, Nelson TJ, Cerhan JR, Roger VL. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol 2013;62:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, Cerhan JR, Roger VL. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol 2016;68:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banke A, Schou M, Videbæk L, Møller JE, Torp-Pedersen C, Gustafsson F, Dahl JS, Køber L, Hildebrandt PR, Gislason GH. Incidence of cancer in patients with chronic heart failure: a long-term follow-up study. Eur J Heart Fail 2016;18:260–266. [DOI] [PubMed] [Google Scholar]
- 10. Rinde LB, Småbrekke B, Hald EM, Brodin EE, Njølstad I, Mathiesen EB, Løchen ML, Wilsgaard T, Brækkan SK, Vik A, Hansen JB. Myocardial infarction and future risk of cancer in the general population—the tromsø study. Eur J Epidemiol 2017;32:193–201. [DOI] [PubMed] [Google Scholar]
- 11. Roderburg C, Loosen SH, Jahn JK, Gänsbacher J, Luedde T, Kostev K, Luedde M. Heart failure is associated with an increased incidence of cancer diagnoses. ESC Hear Fail 2021;8:3628–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leedy DJ, Reding KW, Vasbinder AL, Anderson GL, Barac A, Wactawski-Wende J, Shadyab AH, Eaton CB, Levy WC, Qi LH, Cheng RK. The association between heart failure and incident cancer in women: an analysis of the women’s health initiative. Eur J Heart Fail 2021;23:1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bertero E, Robusto F, Rulli E, D’Ettorre A, Bisceglia L, Staszewsky L, Maack C, Lepore V, Latini R, Ameri P. Cancer incidence and mortality according to pre-existing heart failure in a community-based cohort. JACC CardioOncol 2022;4:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meijers WC, Maglione M, Bakker SJL, Oberhuber R, Kieneker LM, De JS, Haubner BJ, Nagengast WB, Lyon AR, Van Der VB, van Veldhuisen DJ, Westenbrink BD, van der Meer P, Silljé HHW, de Boer RA. Heart failure stimulates tumor growth by circulating factors. Circulation 2018;138:678–691. [DOI] [PubMed] [Google Scholar]
- 15. Koelwyn GJ, Newman AAC, Afonso MS, van Solingen C, Corr EM, Brown EJ, Albers KB, Yamaguchi N, Narke D, Schlegel M, Sharma M, Shanley LC, Barrett TJ, Rahman K, Mezzano V, Fisher EA, Park DS, Newman JD, Quail DF, Nelson ER, Caan BJ, Jones LW, Moore KJ. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat Med 2020;26:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avraham S, Abu-Sharki S, Shofti R, Haas T, Korin B, Kalfon R, Friedman T, Shiran A, Saliba W, Shaked Y, Aronheim A. Early cardiac remodeling promotes tumor growth and metastasis. Circulation 2020;142:670–683. [DOI] [PubMed] [Google Scholar]
- 17. Shi C, Aboumsallem JP, de Wit S, Schouten EM, Bracun V, Meijers WC, Silljé HHW, de Boer RA. Evaluation of renal cancer progression in a mouse model of heart failure. Cancer Commun 2021;41:796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armenian SH, Xu L, Ky B, Sun C, Farol LT, Pal SK, Douglas PS, Bhatia S, Chao C. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol 2016;34:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akbay EA, Moslehi J, Christensen CL, Saha S, Tchaicha JH, Ramkissoon SH, Stewart KM, Carretero J, Kikuchi E, Zhang H, Cohoon TJ, Murray S, Liu W, Uno K, Fisch S, Jones K, Gurumurthy S, Gliser C, Choe S, Keenan M, Son J, Stanley I, Losman JA, Padera R, Bronson RT, Asara JM, Abdel-Wahab O, Amrein PC, Fathi AT, Danial NN, Kimmelman AC, Kung AL, Ligon KL, Yen KE, Kaelin WG, Bardeesy N, Wong KK. D-2-hydroxyglutarate produced by mutant IDH2 causes cardiomyopathy and neurodegeneration in mice. Genes Dev 2014;28:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karlstaedt A, Zhang X, Vitrac H, Harmancey R, Vasquez H, Wang JH, Goodell MA, Taegtmeyer H. Oncometabolite D-2-hydroxyglutarate impairs α-ketoglutarate dehydrogenase and contractile function in rodent heart. Proc Natl Acad Sci U S A 2016;113:10436–10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 22. Anker MS, Sanz AP, Zamorano JL, Mehra MR, Butler J, Riess H, Coats AJS, Anker SD. Advanced cancer is also a heart failure syndrome: a hypothesis. Eur J Heart Fail 2021;23:140–144. [DOI] [PubMed] [Google Scholar]
- 23. Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res 2011;71:1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belloum Y, Rannou-Bekono F, Favier FB. Cancer-induced cardiac cachexia: pathogenesis and impact of physical activity (review). Oncol Rep 2017;37:2543–2552. [DOI] [PubMed] [Google Scholar]
- 25. Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol 2018;15:457–470. [DOI] [PubMed] [Google Scholar]
- 26. DeBerardinis R, Chandel NS. Fundamentals of cancer metabolism. Sci Adv 2016;2:e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcia-Ropero Alvaro, Santos-Gallego Carlos G, Zafar M. Urooj, Badimon Juan J . Metabolism of the failing heart and the impact of SGLT2 inhibitors. Expert Opinion on Drug Metabolism & Toxicology 2019;15:275–285. [DOI] [PubMed] [Google Scholar]
- 28. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev 2015;95:727–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 2013;113:709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol 1994;267:742–750. [DOI] [PubMed] [Google Scholar]
- 31. Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, Shioi T. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Hear Fail 2010;3:420–430. [DOI] [PubMed] [Google Scholar]
- 32. Doenst T, Pytel G, Schrepper A, Amorim P, Färber G, Shingu Y, Mohr FW, Schwarzer M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res 2010;86:461–470. [DOI] [PubMed] [Google Scholar]
- 33. Warburg O. On the origin of cancer cells. Science 1956;123:309–314. [DOI] [PubMed] [Google Scholar]
- 34. Koppenol WH, Bounds PL, Dang C V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 2011;11:325–337. [DOI] [PubMed] [Google Scholar]
- 35. Zhuo B, Li Y, Li Z, Qin H, Sun Q, Zhang F, Shen Y, Shi Y, Wang R. PI3K/Akt Signaling mediated hexokinase-2 expression inhibits cell apoptosis and promotes tumor growth in pediatric osteosarcoma. Biochem Biophys Res Commun 2015;464:401–406. [DOI] [PubMed] [Google Scholar]
- 36. Kim J, Gao P, Liu YC, Semenza GL, Dang C V. Hypoxia-Inducible factor 1 and dysregulated C-MYC cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol 2007;27:7381–7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang C, Liu J, Liang Y, Wu R, Zhao Y, Hong X, Lin M, Yu H, Liu L, Levine AJ, Hu W, Feng Z. Tumour-associated mutant p53 drives the Warburg effect. Nat Commun 2013;4:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beer M, Seyfarth T, Sandstede J, Landschütz W, Lipke C, Köstler H, Von KM, Harre K, Hahn D, Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol 2002;40:1267–1274. [DOI] [PubMed] [Google Scholar]
- 39. Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res 2004;95:135–145. [DOI] [PubMed] [Google Scholar]
- 40. Joshi S, Tolkunov D, Aviv H, Hakimi AA, Yao M, Hsieh JJ, Ganesan S, Chan CS, White E. The genomic landscape of renal oncocytoma identifies a metabolic barrier to tumorigenesis. Cell Rep 2015;13:1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GRS, Chandel NS. Mitochondrial metabolism and ROS generation are essential for kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 2010;107:8788–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW, Burga LN, Xie J, Jurczak MJ, Depinho RA, Clish CB, Jacks T, Kibbey RG, Wulf GM, Di VD, Mills GB, Cantley LC, Vander HM. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell 2013;155:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation 2007;115:2033–2041. [DOI] [PubMed] [Google Scholar]
- 44. Kolwicz SC, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res 2012;111:728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Christen S, Lorendeau D, Schmieder R, Broekaert D, Metzger K, Veys K, Elia I, Buescher JM, Orth MF, Davidson SM, Grünewald TGP, De BK, Fendt SM. Breast cancer-derived lung metastases show increased pyruvate carboxylase-dependent anaplerosis. Cell Rep 2016;17:837–848. [DOI] [PubMed] [Google Scholar]
- 46. Sant’anna-Silva ACB, Perez-Valencia JA, Sciacovelli M, Lalou C, Sarlak S, Tronci L, Nikitopoulou E, Meszaros AT, Frezza C, Rossignol R, Gnaiger E, Klocker H. Succinate anaplerosis has an onco-driving potential in prostate cancer cells. Cancers (Basel) 2021;13:1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lauzier B, Vaillant F, Merlen C, Gélinas R, Bouchard B, Rivard ME, Labarthe F, Dolinsky VW, Dyck JRB, Allen BG, Chatham JC, Des RC. Metabolic effects of glutamine on the heart. Anaplerosis versus the hexosamine biosynthetic pathway. J Mol Cell Cardiol 2013;55:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest 2013;123:3678–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wise DR, Ward PS, Shay JES, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A 2011;108:19611–19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nat 2011;481:380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bedi KC, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016;133:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Ketones and lactate ‘fuel’ tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 2010;9:3506–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rodrigues LM, Uribe-Lewis S, Madhu B, Honess DJ, Stubbsând MS, Griffiths JR. The action of β-hydroxybutyrate on the growth, metabolism and global histone H3 acetylation of spontaneous mouse mammary tumours: evidence of a β-hydroxybutyrate paradox. Cancer Metab 2017;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shukla SK, Gebregiworgis T, Purohit V, Chaika NV, Gunda V, Radhakrishnan P, Mehla K, Pipinos II, Powers R, Yu F, Singh PK. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab 2014;2:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, Szweda LI, Smith BJ, Spitz DR, Fath MA. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res 2013;19:3905–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xiaojun X, Zur HA, Coy JF, Löchelt M. Transketolase-like protein 1 (TKTL1) is required for rapid cell growth and full viability of human tumor cells. Int J Cancer 2009;124:1330–1337. [DOI] [PubMed] [Google Scholar]
- 58. Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, Depinho RA. Oncogenic kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149:656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander HM. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 2015;162:552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene 2005;24:6314–6322. [DOI] [PubMed] [Google Scholar]
- 61. Umapathi P, Mesubi OO, Banerjee PS, Abrol N, Wang Q, Luczak ED, Wu Y, Granger JM, Wei AC, Reyes Gaido OE, Florea L, Talbot CC, Hart GW, Zachara NE, Anderson ME. Excessive O-GlcNAcylation causes heart failure and sudden death. Circulation 2021;143:1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cannon MV, Silljé HH, Sijbesma JW, Vreeswijk-Baudoin I, Ciapaite J, van der Sluis B, van Deursen J, Silva GJ, de Windt LJ, Gustafsson J, van der Harst P, van Gilst WH, de Boer RA. Cardiac LXRα protects against pathological cardiac hypertrophy and dysfunction by enhancing glucose uptake and utilization. EMBO Mol Med 2015;7:1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 2002;8:S62–S67. [DOI] [PubMed] [Google Scholar]
- 64. Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 2010;20:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takasaki C, Kobayashi M, Ishibashi H, Akashi T, Okubo K. Expression of hypoxia-inducible factor-1α affects tumor proliferation and antiapoptosis in surgically resected lung cancer. Mol Clin Oncol 2016;5:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Giles RH, Lolkema MP, Snijckers CM, Belderbos M, Van Der Groep P, Mans DA, Van Beest M, Van Noort M, Goldschmeding R, Van Diest PJ, Clevers H, Voest EE. Interplay between VHL/HIF1α and wnt/β-catenin pathways during colorectal tumorigenesis. Oncogene 2006;25:3065–3070. [DOI] [PubMed] [Google Scholar]
- 67. Simon F, Bockhorn M, Praha C, Baba HA, Broelsch CE, Frilling A, Weber F. Deregulation of HIF1-alpha and hypoxia-regulated pathways in hepatocellular carcinoma and corresponding non-malignant liver tissue-influence of a modulated host stroma on the prognosis of HCC. Langenbecks Arch Surg 2010;395:395–405. [DOI] [PubMed] [Google Scholar]
- 68. Varshney N, Kebede AA, Owusu-Dapaah H, Lather J, Kaushik M, Bhullar JS. A review of Von hippel-lindau syndrome. J Kidney Cancer VHL 2017;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith TG, Brooks JT, Balanos GM, Lappin TR, Layton DM, Leedham DL, Liu C, Maxwell PH, McMullin MF, McNamara CJ, Percy MJ, Pugh CW, Ratcliffe PJ, Talbot NP, Treacy M, Robbins PA. Mutation of the von Hippel-Lindau gene alters human cardiopulmonary physiology. Adv Exp Med Biol 2008;605:51–56. [DOI] [PubMed] [Google Scholar]
- 70. Menendez-Montes I, Escobar B, Palacios B, Gómez MJ, Izquierdo-Garcia JL, Flores L, Jiménez-Borreguero LJ, Aragones J, Ruiz-Cabello J, Torres M, Martin-Puig S. Myocardial VHL-HIF signaling controls an embryonic metabolic switch essential for cardiac maturation. Dev Cell 2016;39:724–739. [DOI] [PubMed] [Google Scholar]
- 71. Lei L, Mason S, Liu D, Huang Y, Marks C, Hickey R, Jovin IS, Pypaert M, Johnson RS, Giordano FJ. Hypoxia-Inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol Cell Biol 2008;28:3790–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, Perriard JC, Larsen T, Pedrazzini T, Krek W. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab 2009;9:512–524. [DOI] [PubMed] [Google Scholar]
- 73. Chu W, Wan L, Zhao D, Qu X, Cai F, Huo R, Wang N, Zhu J, Zhang C, Zheng F, Cai R, Dong D, Lu Y, Yang B. Mild hypoxia-induced cardiomyocyte hypertrophy via up-regulation of HIF-1α-mediated TRPC signalling. J Cell Mol Med 2012;16:2022–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nicks AM, Kesteven SH, Li M, Wu J, Chan AY, Naqvi N, Husain A, Feneley MP, Smith NJ, Iismaa SE, Graham RM. Pressure overload by suprarenal aortic constriction in mice leads to left ventricular hypertrophy without c-kit expression in cardiomyocytes. Sci Reports 2020;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733–794. [DOI] [PubMed] [Google Scholar]
- 76. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Lohlavek JB, Bohm M, Chiang CE, Chopra VK, De BR, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 77. Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Brueckmann M, Jamal W, Zeller C, Schnaidt S, Zannad F. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-reduced trial. Circulation 2021;143:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yurista SR, Silljé HHW, Oberdorf-Maass SU, Schouten EM, Pavez Giani MG, Hillebrands JL, van Goor H, van Veldhuisen DJ, de Boer RA, Westenbrink BD. Sodium–glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail 2019;21:862–873. [DOI] [PubMed] [Google Scholar]
- 79. Connelly KA, Zhang Y, Desjardins JF, Nghiem L, Visram A, Batchu SN, Yerra VG, Kabir G, Thai K, Advani A, Gilbert RE. Load-independent effects of empagliflozin contribute to improved cardiac function in experimental heart failure with reduced ejection fraction. Cardiovasc Diabetol 2020;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Asensio Lopez MDC, Lax A, Hernandez Vicente A, Saura Guillen E, Hernandez-Martinez A, Fernandez del Palacio MJ, Bayes Genis A, Pascual Figal DA. Empagliflozin improves post-infarction cardiac remodeling through GTP enzyme cyclohydrolase 1 and irrespective of diabetes status. Sci Rep 2020;10:13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nasiri AR, Rodrigues MR, Li Z, Leitner BP, Perry RJ. SGLT2 Inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab 2019;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shiba K, Tsuchiya K, Komiya C, Miyachi Y, Mori K, Shimazu N, Yamaguchi S, Ogasawara N, Katoh M, Itoh M, Suganami T, Ogawa Y. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep 2018;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jojima T, Wakamatsu S, Kase M, Iijima T, Maejima Y, Shimomura K, Kogai T, Tomaru T, Usui I, Aso Y. The SGLT2 inhibitor canagliflozin prevents carcinogenesis in a mouse model of diabetes and non-alcoholic steatohepatitis-related hepatocarcinogenesis: association with SGLT2 expression in hepatocellular carcinoma. Int J Mol Sci 2019;20:5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kaji K, Nishimura N, Seki K, Sato S, Saikawa S, Nakanishi K, Furukawa M, Kawaratani H, Kitade M, Moriya K, Namisaki T, Yoshiji H. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer 2018;142:1712–1722. [DOI] [PubMed] [Google Scholar]
- 85. Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, Moatamed NA, Huang J, Koepsell H, Barrio JR, Wright EM. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U S A 2015;112:E4111–E4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Albadari N, Deng S, Li W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin Drug Discov 2019;14:667–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Choueiri TK, Kaelin WG. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat Med 2020;26:1519–1530. [DOI] [PubMed] [Google Scholar]
- 88. Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, Oudard S, Else T, Maranchie JK, Welsh SJ, Thamake S, Park EK, Perini RF, Linehan WM, Srinivasan R. Belzutifan for renal cell carcinoma in von Hippel–Lindau disease. N Engl J Med 2021;385:2036–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Oh SY, Seok JY, Choi YS, Lee SH, Bae JS, Lee YM. The histone methyltransferase inhibitor BIX01294 inhibits HIF-1α stability and angiogenesis. Mol Cells 2015;38:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Parhira S, Zhu GY, Chen M, Bai LP, Jiang ZH. Cardenolides from Calotropis gigantea as potent inhibitors of hypoxia-inducible factor-1 transcriptional activity. J Ethnopharmacol 2016;194:930–936. [DOI] [PubMed] [Google Scholar]
- 91. Liu Y, Zou J, Liu X, Zhang Q. MicroRNA-138 attenuates myocardial ischemia reperfusion injury through inhibiting mitochondria-mediated apoptosis by targeting HIF1-α. Exp Ther Med 2019;18:3325–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ghosh MC, Zhang DL, Ollivierre WH, Noguchi A, Springer DA, Linehan WM, Rouault TA. Therapeutic inhibition of HIF-2α reverses polycythemia and pulmonary hypertension in murine models of human diseases. Blood 2021;137:2509–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990;323:236–241. [DOI] [PubMed] [Google Scholar]
- 94. Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol 2020;17:269–285. [DOI] [PubMed] [Google Scholar]
- 95. Coussens LM, Werb Z. Inflammation and cancer. Nat 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014;2014:149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, Schleck CD, Tremaine WJ, Melton LJ, Munkholm P, Sandborn WJ. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from Olmsted county, Minnesota. Gastroenterology 2006;130:1039–1046. [DOI] [PubMed] [Google Scholar]
- 98. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264–1273.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ungprasert P, Charoenpong P, Ratanasrimetha P, Thongprayoon C, Cheungpasitporn W, Suksaranjit P. Risk of coronary artery disease in patients with systemic sclerosis: a systematic review and meta-analysis. Clin Rheumatol 2014;33:1099–1104. [DOI] [PubMed] [Google Scholar]
- 100. Hilfiker-Kleiner D, Landmesser U, Drexler H. Molecular mechanisms in heart failure: focus on cardiac hypertrophy, inflammation, angiogenesis, and apoptosis. J Am Coll Cardiol 2006;48:A56–A66. [Google Scholar]
- 101. Shimizu I, Yoshida Y, Katsuno T, Minamino T. Adipose tissue inflammation in diabetes and heart failure. Microbes Infect 2013;15:11–17. [DOI] [PubMed] [Google Scholar]
- 102. Tselepis C, Perry I, Dawson C, Hardy R, Darnton SJ, McConkey C, Stuart RC, Wright N, Harrison R, Jankowski JAZ. Tumour necrosis factor-α in Barrett’s oesophagus: a potential novel mechanism of action. Oncogene 2002;21:6071–6081. [DOI] [PubMed] [Google Scholar]
- 103. Gasche JA, Hoffmann J, Boland CR, Goel A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer 2011;129:1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012;2012:948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Thomas DA, Massagué J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005;8:369–380. [DOI] [PubMed] [Google Scholar]
- 106. Oikawa T, Sakata Y, Nochioka K, Miura M, Abe R, Kasahara S, Sato M, Aoyanagi H, Shiroto T, Sugimura K, Takahashi J, Miyata S, Shimokawa H. Increased risk of cancer death in patients with chronic heart failure with a special reference to inflammation-A report from the CHART-2 study. Int J Cardiol 2019;290:106–112. [DOI] [PubMed] [Google Scholar]
- 107. Van’t Klooster CC, Ridker PM, Hjortnaes J, Van Der GY, Asselbergs FW, Westerink J, Aerts JGJV, Visseren FLJ, Asselbergs FW, Nathoe HM, De BG, Bots ML, Geerlings MI, Emmelot MH, De JP, Leiner T, Lely AT, Van Der KN, Kappelle LJ, Ruigrok Y, Verhaar MC, Visseren FLJ, Westerink J. The relation between systemic inflammation and incident cancer in patients with stable cardiovascular disease: a cohort study. Eur Heart J 2019;40:3901–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. DeZern AE, Malcovati L, Ebert BL. CHIP, CCUS, and other acronyms: definition, implications, and impact on practice. Am Soc Clin Oncol Educ book 2019;39:400–410. [DOI] [PubMed] [Google Scholar]
- 109. Mooney L, Goodyear CS, Chandra T, Kirschner K, Copland M, Petrie MC, Lang NN. Clonal haematopoiesis of indeterminate potential: intersections between inflammation, vascular disease and heart failure. Clin Sci (Lond) 2021;135:991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Saiki R, Momozawa Y, Nannya Y, Nakagawa MM, Ochi Y, Yoshizato T, Terao C, Kuroda Y, Shiraishi Y, Chiba K, Tanaka H, Niida A, Imoto S, Matsuda K, Morisaki T, Murakami Y, Kamatani Y, Matsuda S, Kubo M, Miyano S, Makishima H, Ogawa S. Combined landscape of single-nucleotide variants and copy number alterations in clonal hematopoiesis. Nat Med 2021;27:1239–1249. [DOI] [PubMed] [Google Scholar]
- 112. Bhattacharya R, Zekavat SM, Haessler J, Fornage M, Raffield L, Uddin MM, Bick AG, Niroula A, Yu B, Gibson C, Griffin G, Morrison AC, Psaty BM, Longstreth WT, Bis JC, Rich SS, Rotter JI, Tracy RP, Correa A, Seshadri S, Johnson A, Collins JM, Hayden KM, Madsen TE, Ballantyne CM, Jaiswal S, Ebert BL, Kooperberg C, Manson JE, Whitsel EA, Natarajan P, Reiner AP. Clonal hematopoiesis is associated with higher risk of stroke. Stroke 2022;53:788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K, Schmid T, Brüne B, Wagner S, Serve H, Hoffmann J, Seeger F, Dimmeler S, Zeiher AM, Rieger MA. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol 2019;4:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pascual-Figal DA, Bayes-Genis A, Díez-Díez M, Hernández-Vicente Á, Vázquez-Andrés D, de la Barrera J, Vazquez E, Quintas A, Zuriaga MA, Asensio-López MC, Dopazo A, Sánchez-Cabo F, Fuster JJ. Clonal hematopoiesis and risk of progression of heart failure with reduced left ventricular ejection fraction. J Am Coll Cardiol 2021;77:1747–1759. [DOI] [PubMed] [Google Scholar]
- 115. Yu B, Roberts MB, Raffield LM, Zekavat SM, Nguyen NQH, Biggs ML, Brown MR, Griffin G, Desai P, Correa A, Morrison AC, Shah AM, Niroula A, Uddin MM, Honigberg MC, Ebert BL, Psaty BM, Whitsel EA, Manson JAE, Kooperberg C, Bick AG, Ballantyne CM, Reiner AP, Natarajan P, Eaton CB. Supplemental association of clonal hematopoiesis with incident heart failure. J Am Coll Cardiol 2021;78:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cook EK, Izukawa T, Young S, Rosen G, Jamali M, Zhang L, Johnson D, Bain E, Hilland J, Ferrone CK, Buckstein J, Francis J, Momtaz B, McNaughton AJM, Liu X, Snetsinger B, Buckstein R, Rauh MJ. Comorbid and inflammatory characteristics of genetic subtypes of clonal hematopoiesis. Blood Adv 2019;3:2482–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bick AG, Weinstock JS, Nandakumar SK, Fulco CP, Bao EL, Zekavat SM, Szeto MD, Liao X, Leventhal MJ, Nasser J, Chang K, Laurie C, Burugula BB, Gibson CJ, Lin AE, Taub MA, Aguet F, Ardlie K, Mitchell BD, Barnes KC, Moscati A, Fornage M, Redline S, Psaty BM, Silverman EK, Weiss ST, Palmer ND, Vasan RS, Burchard EG, Kardia SLR, He J, Kaplan RC, Smith NL, Arnett DK, Schwartz DA, Correa A, de Andrade M, Guo X, Konkle BA, Custer B, Peralta JM, Gui H, Meyers DA, McGarvey ST, Chen IY, Shoemaker MB, Peyser PA, Broome JG, Gogarten SM, Wang FF, Wong Q, Montasser ME, Daya M, Kenny EE, North KE, Launer LJ, Cade BE, Bis JC, Cho MH, Lasky-Su J, Bowden DW, Cupples LA, Mak ACY, Becker LC, Smith JA, Kelly TN, Aslibekyan S, Heckbert SR, Tiwari HK, Yang IV, Heit JA, Lubitz SA, Johnsen JM, Curran JE, Wenzel SE, Weeks DE, Rao DC, Darbar D, Moon JY, Tracy RP, Buth EJ, Rafaels N, Loos RJF, Durda P, Liu Y, Hou L, Lee J, Kachroo P, Freedman BI, Levy D, Bielak LF, Hixson JE, Floyd JS, Whitsel EA, Ellinor PT, Irvin MR, Fingerlin TE, Raffield LM, Armasu SM, Wheeler MM, Sabino EC, Blangero J, Williams LK, Levy BD, Sheu WH, Roden DM, Boerwinkle E, Manson JE, Mathias RA, Desai P, Taylor KD, Johnson AD; NHLBI Trans-Omics for Precision Medicine Consortium, Auer PL, Kooperberg C, Laurie CC, Blackwell TW, Smith AV, Zhao H, Lange E, Lange L, Rich SS, Rotter JI, Wilson JG, Scheet P, Kitzman JO, Lander ES, Engreitz JM, Ebert BL, Reiner AP, Jaiswal S, Abecasis G, Sankaran VG, Kathiresan S, Natarajan P. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nat 2020;586:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, Walsh K. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardiol 2018;71:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K. CRISPR-mediated gene editing to assess the roles of TET2 and DNMT3A in clonal hematopoiesis and cardiovascular disease. Circ Res 2018;123:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sano S, Wang Y, Yura Y, Sano M, Oshima K, Yang Y, Katanasaka Y, Min KD, Matsuura S, Ravid K, Mohi G, Walsh K. JAK2V617F-mediated clonal hematopoiesis accelerates pathological remodeling in murine heart failure. JACC Basic to Transl Sci 2019;4:684–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. WHO . Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (6 April 2022).
- 122. Cipolletta D. Adipose tissue-resident regulatory T cells: phenotypic specialization, functions and therapeutic potential. Immunology 2014;142:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011;332:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity 2014;41:36–48. [DOI] [PubMed] [Google Scholar]
- 125. Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, Ferlay J, Miranda JJ, Romieu I, Dikshit R, Forman D, Soerjomataram I. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol 2015;16:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med 2016;375:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, He X, Fuchs CS, Ogino S, Willett WC, Chan AT, Giovannucci EL, Cao Y. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol 2019;5:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 129. Elangovan A, Skeans J, Landsman M, Ali SMJ, Elangovan AG, Kaelber DC, Sandhu DS, Cooper GS. Colorectal cancer, age, and obesity-related comorbidities: a large database study. Dig Dis Sci 2021;66:3156–3163. [DOI] [PubMed] [Google Scholar]
- 130. Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol Mech Dis 2016;11:421–449. [DOI] [PubMed] [Google Scholar]
- 131. Mercurio V, Cuomo A, Dessalvi CC, Deidda M, Di LD, Novo G, Manganaro R, Zito C, Santoro C, Ameri P, Spallarossa P, Arboscello E, Tocchetti CG, Penna C. Redox imbalances in ageing and metabolic alterations: implications in cancer and cardiac diseases. An overview from the working group of cardiotoxicity and cardioprotection of the Italian Society of Cardiology (SIC). Antioxidants 2020;9:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le GS, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer InvasionKey role of mature adipocytes in breast cancer progression. Cancer Res 2011;71:2455–2465. [DOI] [PubMed] [Google Scholar]
- 133. Atish S, Enchaiah K, Ane J, Vans CE, Evy AL, Ilson EWFW, Enjamin MJB, Arson AGL, Annel IBK, Amachandran R, Asan S V. Obesity and the risk of heart failure. N Engl J Med 2002;35:59–60. [Google Scholar]
- 134. Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, Nambi V, Ballantyne CM, Solomon SD, Selvin E, Folsom AR, Coresh J. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation 2016;133:639–649. [DOI] [PubMed] [Google Scholar]
- 136. Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Hear Fail 2018;6:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Alex L, Russo I, Holoborodko V, Frangogiannis NG. Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am J Physiol - Hear Circ Physiol 2018;315:H934–H949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vlasova M, Purhonen AK, Jarvelin MR, Rodilla E, Pascual J, Herzig KH. Role of adipokines in obesity-associated hypertension. Acta Physiol 2010;200:107–127. [DOI] [PubMed] [Google Scholar]
- 139. Ntaios G, Gatselis NK, Makaritsis K, Dalekos GN. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis 2013;227:216–221. [DOI] [PubMed] [Google Scholar]
- 140. Pello Lázaro AM, Blanco-Colio LM, Franco Peláez JA, Tuñón J. Anti-Inflammatory drugs in patients with ischemic heart disease. J Clin Med 2021;10:2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 142. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 143. Ridker PM, MacFadyen JG, Thuren T, Everett B, Libby P, Glynn R, Ridker P, Lorenzatti A, Krum H, Varigos J, Siostrzonek P, Sinnaeve P, Fonseca F, Nicolau J, Gotcheva N, Genest J, Yong H, Urina-Triana M, Milicic D, Cifkova R, Vettus R, Koenig W, Anker SD, Manolis AJ, Wyss F, Forster T, Sigurdsson A, Pais P, Fucili A, Ogawa H, Shimokawa H, Veze I, Petrauskiene B, Salvador L, Kastelein J, Cornel JH, Klemsdal TO, Medina F, Budaj A, Vida-Simiti L, Kobalava Z, Otasevic P, Pella D, Lainscak M, Seung KB, Commerford P, Dellborg M, Donath M, Hwang JJ, Kultursay H, Flather M, Ballantyne C, Bilazarian S, Chang W, East C, Forgosh L, Harris B, Ligueros M, Bohula E, Charmarthi B, Cheng S, Chou S, Danik J, McMahon G, Maron B, Ning MM, Olenchock B, Pande R, Perlstein T, Pradhan A, Rost N, Singhal A, Taqueti V, Wei N, Burris H, Cioffi A, Dalseg AM, Ghosh N, Gralow J, Mayer T, Rugo H, Fowler V, Limaye AP, Cosgrove S, Levine D, Lopes R, Scott J, Hilkert R, Tamesby G, Mickel C, Manning B, Woelcke J, Tan M, Manfreda S, Ponce T, Kam J, Saini R, Banker K, Salko T, Nandy P, Tawfik R, O’Neil G, Manne S, Jirvankar P, Lal S, Nema D, Jose J, Collins R, Bailey K, Blumenthal R, Colhoun H, Gersh B. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]
- 144. Garrido P, Pujol JL, Kim ES, Lee JM, Tsuboi M, Gómez-Rueda A, Benito A, Moreno N, Gorospe L, Dong T, Blin C, Rodrik-Outmezguine V, Passos VQ, Mok TSK. Canakinumab with and without pembrolizumab in patients with resectable non-small-cell lung cancer: CANOPY-N study design. Futur Oncol 2021;17:1459–1472. [DOI] [PubMed] [Google Scholar]
- 145. Hussain M, Adah D, Tariq M, Lu Y, Zhang J, Liu J. CXCL13/CXCR5 signaling axis in cancer. Life Sci 2019;227:175–186. [DOI] [PubMed] [Google Scholar]
- 146. Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 As an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia 2007;9:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Egerstedt A, Berntsson J, Smith ML, Gidlöf O, Nilsson R, Benson M, Wells QS, Celik S, Lejonberg C, Farrell L, Sinha S, Shen D, Lundgren J, Rådegran G, Ngo D, Engström G, Yang Q, Wang TJ, Gerszten RE, Smith JG. Profiling of the plasma proteome across different stages of human heart failure. Nat Commun 2019;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hohensinner PJ, Rychli K, Zorn G, Hülsmann M, Berger R, Mörtl D, Richter B, Huber K, Wojta J, Pacher R, Niessner A. Macrophage-modulating cytokines predict adverse outcome in heart failure. Thromb Haemost 2010;103:435–441. [DOI] [PubMed] [Google Scholar]
- 149. Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 2005;96:881–889. [DOI] [PubMed] [Google Scholar]
- 150. Zhu Z, Zhang X, Guo H, Fu L, Pan G, Sun Y. CXCL13-CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway. Mol Cell Biochem 2015;400:287–295. [DOI] [PubMed] [Google Scholar]
- 151. Xu L, Liang Z, Li S, Ma J. Signaling via the CXCR5/ERK pathway is mediated by CXCL13 in mice with breast cancer. Oncol Lett 2018;15:9293–9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hao Q, Vadgama J V, Wang P. CCL2/CCR2 Signaling in cancer pathogenesis. Cell Commun Signal 2020;18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Teng KY, Han J, Zhang X, Hsu SH, He S, Wani NA, Barajas JM, Snyder LA, Frankel WL, Caligiuri MA, Jacob ST, Yu J, Ghoshal K. Blocking the CCL2-CCR2 axis using CCL2-neutralizing antibody is an effective therapy for hepatocellular cancer in a mouse model. Mol Cancer Ther 2017;16:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Xu M, Wang Y, Xia R, Wei Y, Wei X. Role of the CCL2-CCR2 signalling axis in cancer: mechanisms and therapeutic targeting. Cell Prolif 2021;54:e13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Hanna A, Frangogiannis NG. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc Drugs Ther 2020;34:849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56–61. [DOI] [PubMed] [Google Scholar]
- 157. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol 2021;16:223–249. [DOI] [PubMed] [Google Scholar]
- 158. Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir-Riahi A, Tawbi H, Pauschinger M, Gajewski TF, Lipson EJ, Luke JJ. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 2016;4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Rapid increase in reporting of fatal immune checkpoint inhibitor associated myocarditis. Lancet 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology 2009;136:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 2005;307:1915–1920. [DOI] [PubMed] [Google Scholar]
- 163. Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ 2018;361:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, Shilo S, Lador D, Vila AV, Zmora N, Pevsner-Fischer M, Israeli D, Kosower N, Malka G, Wolf BC, Avnit-Sagi T, Lotan-Pompan M, Weinberger A, Halpern Z, Carmi S, Fu J, Wijmenga C, Zhernakova A, Elinav E, Segal E. Environment dominates over host genetics in shaping human gut microbiota. Nat 2018;555:210–215. [DOI] [PubMed] [Google Scholar]
- 165. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nat 2008;457:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, Goedert JJ, Hayes RB, Yang L. Human gut microbiome and risk for colorectal cancer. JNCI J Natl Cancer Inst 2013;105:1907–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lu Y, Chen J, Zheng J, Hu G, Wang J, Huang C, Lou L, Wang X, Zeng Y. Mucosal adherent bacterial dysbiosis in patients with colorectal adenomas. Sci Rep 2016;6:26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wu N, Yang X, Zhang R, Li J, Xiao X, Hu Y, Chen Y, Yang F, Lu N, Wang Z, Luan C, Liu Y, Wang B, Xiang C, Wang Y, Zhao F, Gao GF, Wang S, Li L, Zhang H, Zhu B. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb Ecol 2013;66:462–470. [DOI] [PubMed] [Google Scholar]
- 169. Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, Xiao X, Kwong TNY, Tsoi H, Wu WKK, Zeng B, Chan FKL, Sung JJY, Wei H, Yu J. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology 2017;153:1621–1633.e6. [DOI] [PubMed] [Google Scholar]
- 170. Li L, Li X, Zhong W, Yang M, Xu M, Sun Y, Ma J, Liu T, Song X, Dong W, Liu X, Chen Y, Liu Y, Abla Z, Liu W, Wang B, Jiang K, Cao H. Gut microbiota from colorectal cancer patients enhances the progression of intestinal adenoma in apc min/+ mice. EBioMedicine 2019;48:301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Jain T, Sharma P, Are AC, Vickers SM, Dudeja V. New insights into the cancer–microbiome–immune axis: decrypting a decade of discoveries. Front Immunol 2021;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science 2021;371:eabc4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Luedde M, Winkler T, Heinsen FA, Rühlemann MC, Spehlmann ME, Bajrovic A, Lieb W, Franke A, Ott SJ, Frey N. Heart failure is associated with depletion of core intestinal microbiota. ESC Hear Fail 2017;4:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Beale AL, O’Donnell JA, Nakai ME, Nanayakkara S, Vizi D, Carter K, Dean E, Ribeiro RV, Yiallourou S, Carrington MJ, Marques FZ, Kaye DM. The gut microbiome of heart failure with preserved ejection fraction. J Am Heart Assoc 2021;10:20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kummen M, Mayerhofer CCK, Vestad B, Broch K, Awoyemi A, Storm-Larsen C, Ueland T, Yndestad A, Hov JR, Trøseid M. Gut Microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol 2018;71:1184–1186. [DOI] [PubMed] [Google Scholar]
- 176. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, Didonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WHW, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Brandsma E, Kloosterhuis NJ, Koster M, Dekker DC, Gijbels MJJ, van der Velden S, Ríos-Morales M, van Faassen MJR, Loreti MG, de Bruin A, Fu J, Kuipers F, Bakker BM, Westerterp M, de Winther MPJ, Hofker MH, van de Sluis B, Koonen DPY. A proinflammatory gut Microbiota increases systemic inflammation and accelerates atherosclerosis. Circ Res 2019;124:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, Ou C, Chen M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Investig 2018;99:346–357. [DOI] [PubMed] [Google Scholar]
- 179. Zheng A, Yi H, Li F, Han L, Yu J, Cheng X, Su H, Hong K, Juxiang L. Changes in gut microbiome structure and function of rats with isoproterenol-induced heart failure. Int Heart J 2019;60:1176–1183. [DOI] [PubMed] [Google Scholar]
- 180. Carrillo-Salinas FJ, Anastasiou M, Ngwenyama N, Kaur K, Tai A, Smolgovsky SA, Jetton D, Aronovitz M, Alcaide P. Gut dysbiosis induced by cardiac pressure overload enhances adverse cardiac remodeling in a T cell-dependent manner. Gut Microbes 2020;12:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Caparrós-Martín JA, Lareu RR, Ramsay JP, Peplies J, Reen FJ, Headlam HA, Ward NC, Croft KD, Newsholme P, Hughes JD, O’gara F. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, Wang J, Imhann F, Brandsma E, Jankipersadsing SA, Joossens M, Cenit MC, Deelen P, Swertz MA, Weersma RK, Feskens EJM, Netea MG, Gevers D, Jonkers D, Franke L, Aulchenko YS, Huttenhower C, Raes J, Hofker MH, Xavier RJ, Wijmenga C, Fu J. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016;352:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Tuteja S, Ferguson JF. Gut microbiome and response to cardiovascular drugs. Circ Genomic Precis Med 2019;12:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology 2009;136:1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Alon-Maimon T, Mandelboim O, Bachrach G. Fusobacterium nucleatum and cancer. Periodontol 2000 2022;00:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Zhang S, Li C, Liu J, Geng F, Shi X, Li Q, Lu Z, Pan Y. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J 2020;287:4032–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wu S, Powell J, Mathioudakis N, Kane S, Fernandez E, Sears CL. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-κB pathway. Infect Immun 2004;72:5832–5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Wu S, Morin PJ, Maouyo D, Sears CL. Bacteroides fragilis enterotoxin induces C-MYC expression and cellular proliferation. Gastroenterology 2003;124:392–400. [DOI] [PubMed] [Google Scholar]
- 189. Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC, Platz EA, Pardoll DM, Sears CL. The Bacteroides fragilis toxin gene is prevalent in the colon Mucosa of colorectal cancer patients. Clin Infect Dis 2015;60:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, Verri M, Dioguardi F. Pathogenic gut flora in patients with chronic heart failure. JACC Hear Fail 2016;4:220–227. [DOI] [PubMed] [Google Scholar]
- 191. Pastori D, Carnevale R, Nocella C, Novo M, Santulli M, Cammisotto V, Menichelli D, Pignatelli P, Violi F. Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: effect of adherence to Mediterranean diet. J Am Heart Assoc 2017;6:e005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Lew WYW, Bayna E, Molle ED, Dalton ND, Lai NC, Bhargava V, Mendiola V, Clopton P, Tang T. Recurrent exposure to subclinical lipopolysaccharide increases mortality and induces cardiac fibrosis in mice. PLoS One 2013;8:e61057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Mao S, Ma H, Chen P, Liang Y, Zhang M, Hinek A. Fat-1 transgenic mice rich in endogenous omega-3 fatty acids are protected from lipopolysaccharide-induced cardiac dysfunction. ESC Hear Fail 2021;8:1966–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol 2019;16:137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res 2020;127:553–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Trøseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjørndal B, Halvorsen B, Karlsen TH, Aukrust P, Gullestad L, Berge RK, Yndestad A. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med 2015;277:717–726. [DOI] [PubMed] [Google Scholar]
- 197. Tang WHW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 2014;64:1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Suzuki T, Heaney LM, Bhandari SS, Jones DJL, Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Heart 2016;102:841–848. [DOI] [PubMed] [Google Scholar]
- 199. Schuett K, Kleber ME, Scharnagl H, Lorkowski S, März W, Niessner A, Marx N, Meinitzer A. Trimethylamine-N-oxide and heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2017;70:3202–3204. [DOI] [PubMed] [Google Scholar]
- 200. Bae S, Ulrich CM, Neuhouser ML, Malysheva O, Bailey LB, Xiao L, Brown EC, Cushing-Haugen KL, Zheng Y, Cheng TYD, Miller JW, Green R, Lane DS, Beresford SAA, Caudill MA. Plasma choline metabolites and colorectal cancer risk in the women’s health initiative observational study. Cancer Res 2014;74:7442–7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Liu X, Liu H, Yuan C, Zhang Y, Wang W, Hu S, Liu L, Wang Y. Preoperative serum TMAO level is a new prognostic marker for colorectal cancer. Biomark Med 2017;11:443–447. [DOI] [PubMed] [Google Scholar]
- 202. Fu BC, Hullar MAJ, Randolph TW, Franke AA, Monroe KR, Cheng I, Wilkens LR, Shepherd JA, Madeleine MM, Le ML, Lim U, Lampe JW. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the multiethnic cohort adiposity phenotype study. Am J Clin Nutr 2020;111:1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Guertin KA, Li XS, Graubard BI, Albanes D, Weinstein SJ, Goedert JJ, Wang Z, Hazen SL, Sinha R. Serum trimethylamine n-oxide, carnitine, choline, and betaine in relation to colorectal cancer risk in the alpha tocopherol, beta carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev 2017;26:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Mondul AM, Moore SC, Weinstein SJ, Karoly ED, Sampson JN, Albanes D. Metabolomic analysis of prostate cancer risk in a prospective cohort: the alpha-tocopherol, beta-carotene cancer prevention (ATBC) study. Int J Cancer 2015;137:2124–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Bag S, Banerjee DR, Basak A, Das AK, Pal M, Banerjee R, Paul RR, Chatterjee J. NMR (1h and 13C) based signatures of abnormal choline metabolism in oral squamous cell carcinoma with no prominent Warburg effect. Biochem Biophys Res Commun 2015;459:574–578. [DOI] [PubMed] [Google Scholar]
- 206. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, Li Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 2021;6:1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J 2012;6:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, Zhang D, Su Z, Fang Z, Lan Z, Li J, Xiao L, Li J, Li R, Li X, Li F, Ren H, Huang Y, Peng Y, Li G, Wen B, Dong B, Chen JY, Geng QS, Zhang ZW, Yang H, Wang J, Wang J, Zhang X, Madsen L, Brix S, Ning G, Xu X, Liu X, Hou Y, Jia H, He K, Kristiansen K. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 2017;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Liu P, Wang Y, Yang G, Zhang Q, Meng L, Xin Y, Jiang X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res 2021;165:105420. [DOI] [PubMed] [Google Scholar]
- 211. Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell 2012;48:612–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Gonçalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab 2013;14:994–1008. [DOI] [PubMed] [Google Scholar]
- 213. Mirzaei R, Afaghi A, Babakhani S, Sohrabi MR, Hosseini-Fard SR, Babolhavaeji K, Khani Ali Akbari S, Yousefimashouf R, Karampoor S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed Pharmacother 2021;139:111619. [DOI] [PubMed] [Google Scholar]
- 214. Luo S, Li Z, Mao L, Chen S, Sun S. Sodium butyrate induces autophagy in colorectal cancer cells through LKB1/AMPK signaling. J Physiol Biochem 2019;75:53–63. [DOI] [PubMed] [Google Scholar]
- 215. Zeng H, Taussig DP, Cheng WH, Johnson LAK, Hakkak R. Butyrate inhibits cancerous HCT116 colon cell proliferation but to a lesser extent in noncancerous NCM460 colon cells. Nutr 2017;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res 2017;120:1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kirschner SK, Deutz NEP, Rijnaarts I, Smit TJ, Larsen DJ, Engelen MPKJ. Impaired intestinal function is associated with lower muscle and cognitive health and well-being in patients with congestive heart failure. J Parenter Enter Nutr 2022;46:660–670. [DOI] [PubMed] [Google Scholar]
- 218. Russo M, Guida F, Paparo L, Trinchese G, Aitoro R, Avagliano C, Fiordelisi A, Napolitano F, Mercurio V, Sala V, Li M, Sorriento D, Ciccarelli M, Ghigo A, Hirsch E, Bianco R, Iaccarino G, Abete P, Bonaduce D, Calignano A, Berni Canani R, Tocchetti CG. The novel butyrate derivative phenylalanine-butyramide protects from doxorubicin-induced cardiotoxicity. Eur J Heart Fail 2019;21:519–528. [DOI] [PubMed] [Google Scholar]
- 219. Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, Haase N, Kräker K, Hering L, Maase M, Kusche-Vihrog K, Grandoch M, Fielitz J, Kempa S, Gollasch M, Zhumadilov Z, Kozhakhmetov S, Kushugulova A, Eckardt KU, Dechend R, Rump LC, Forslund SK, Müller DN, Stegbauer J, Wilck N. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 2019;139:1407–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Zhang L, Du J, Yano N, Wang H, Zhao YT, Dubielecka PM, Zhuang S, Chin YE, Qin G, Zhao TC. Sodium butyrate protects against high fat diet-induced cardiac dysfunction and metabolic disorders in type II diabetic mice. J Cell Biochem 2017;118:2395–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. olfactory receptor responding to gut microbiotaderived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A 2013;110:4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019;321:288–300. [DOI] [PubMed] [Google Scholar]
- 223. Chen T, Xu T, Li Y, Liang C, Chen J, Lu Y, Wu Z, Wu S. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer Treat Rev 2011;37:312–320. [DOI] [PubMed] [Google Scholar]
- 224. Bowles EJA, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KAB, Davis RL, Habel LA, Yood MU, McCarty C, Magid DJ, Wagner EH. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 2012;104:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Lemmens K, Doggen K, De KG. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation 2007;116:954–960. [DOI] [PubMed] [Google Scholar]
- 226. Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Chien KR, Lee KF. Erbb2 is essential in the prevention of dilated cardiomyopathy. Nat Med 2002;8:459–465. [DOI] [PubMed] [Google Scholar]
- 227. Mercurio V, Pirozzi F, Lazzarini E, Marone G, Rizzo P, Agnetti G, Tocchetti CG, Ghigo A, Ameri P. Models of heart failure based on the cardiotoxicity of anticancer drugs. J Card Fail 2016;22:449–458. [DOI] [PubMed] [Google Scholar]
- 228. Vermeulen Z, Segers VFM, De KG. Erbb2 signaling at the crossing between heart failure and cancer. Basic Res Cardiol 2016;111:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med 2007;357:39–51. [DOI] [PubMed] [Google Scholar]
- 230. Gordon LI, Burke MA, Singh ATK, Prachand S, Lieberman ED, Sun L, Naik TJ, Prasad SV N, Ardehali H. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen Species-dependent pathways. J Biol Chem 2009;284:2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Wu Q, Bai B, Tian C, Li D, Yu H, Song B, Li B, Chu X. The molecular mechanisms of cardiotoxicity induced by HER2, VEGF, and tyrosine kinase inhibitors: an updated review. Cardiovasc Drugs Ther 2021;2021:1–14. [DOI] [PubMed] [Google Scholar]
- 232. Mohan N, Shen Y, Endo Y, ElZarrad MK, Wu WJ. Trastuzumab, but not pertuzumab, dysregulates HER2 signaling to mediate inhibition of autophagy and increase in reactive oxygen species production in human cardiomyocytes. Mol Cancer Ther 2016;15:1321–1331. [DOI] [PubMed] [Google Scholar]
- 233. Small HY, Montezano AC, Rios FJ, Savoia C, Touyz RM. Hypertension due to antiangiogenic cancer therapy with vascular endothelial growth factor inhibitors: understanding and managing a new syndrome. Can J Cardiol 2014;30:534–543. [DOI] [PubMed] [Google Scholar]
- 234. Van DD, Dobbin SJH, Neves KB, Herrmann J, Herrmann SM, Versmissen J, Mathijssen RHJ, Danser AHJ, Lang NN. Hypertension and prohypertensive antineoplastic therapies in cancer patients. Circ Res 2021;131:1040–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Ghatalia P, Morgan CJ, Je Y, Nguyen PL, Trinh QD, Choueiri TK, Sonpavde G. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol 2015;94:228–237. [DOI] [PubMed] [Google Scholar]
- 236. Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 2016;17:611–625. [DOI] [PubMed] [Google Scholar]
- 237. Zheng W, Seftor EA, Meininger CJ, Hendrix MJC, Tomanek RJ. Mechanisms of coronary angiogenesis in response to stretch: role of vegf and tgf-β. Am J Physiol Hear Circ Physiol 2001;280:H909-17. [DOI] [PubMed] [Google Scholar]
- 238. Levy AP, Levy NS, Loscalzo J, Calderone A, Takahashi N, Yeo KT, Koren G, Colucci WS, Goldberg MA. Regulation of vascular endothelial growth factor in cardiac myocytes. Circ Res 1995;76:758–766. [DOI] [PubMed] [Google Scholar]
- 239. Hahn VS, Zhang KW, Sun L, Narayan V, Lenihan DJ, Ky B. Heart failure with targeted cancer therapies: mechanisms and cardioprotection. Circ Res 2021;128:1576–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Dobbin SJH, Mangion K, Berry C, Roditi G, Basak S, Sourbron S, White J, Venugopal B, Touyz RM, Jones RJ, Petrie MC, Lang NN. Cardiotoxicity and myocardial hypoperfusion associated with anti-vascular endothelial growth factor therapies: prospective cardiac magnetic resonance imaging in patients with cancer. Eur J Heart Fail 2020;22:1276–1277. [DOI] [PubMed] [Google Scholar]
- 241. Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer 2007;7:332–344. [DOI] [PubMed] [Google Scholar]
- 242. Dobbin SJH, Petrie MC, Myles RC, Touyz RM, Lang NN. Cardiotoxic effects of angiogenesis inhibitors. Clin Sci 2021;135:71–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Akbani R, Akdemir KC, Aksoy BA, Albert M, Ally A, Amin SB, Arachchi H, Arora A, Auman JT, Ayala B, Baboud J, Balasundaram M, Balu S, Barnabas N, Bartlett J, Bartlett P, Bastian BC, Baylin SB, Behera M, Belyaev D, Benz C, Bernard B, Beroukhim R, Bir N, Black AD, Bodenheimer T, Boice L, Boland GM, Bono R, Bootwalla MS, Bosenberg M, Bowen J, Bowlby R, Bristow CA, Brockway-Lunardi L, Brooks D, Brzezinski J, Bshara W, Buda E, Burns WR, Butterfield YSN, Button M, Calderone T, Cappellini GA, Carter C, Carter SL, Cherney L, Cherniack AD, Chevalier A, Chin L, Cho J, Cho RJ, La CY, Chu A, Chudamani S, Cibulskis K, Ciriello G, Clarke A, Coons S, Cope L, Crain D, Curley E, Danilova L, D’Atri S, Davidsen T, Davies MA, Delman KA, Demchok JA, Deng QA, Deribe YL, Dhalla N, Dhir R, Dicara D, Dinikin M, Dubina M, Ebrom JS, Egea S, Eley G, Engel J, Eschbacher JM, Fedosenko K V, Felau I, Fennell T, Ferguson ML, Fisher S, Flaherty KT, Frazer S, Frick J, Fulidou V, Gabriel SB, Gao J, Gardner J, Garraway LA, Gastier-Foster JM, Gaudioso C, Gehlenborg N, Genovese G, Gerken M, Gershenwald JE, Getz G, Gomez-Fernandez C, Gribbin T, Grimsby J, Gross B, Guin R, Gutschner T, Hadjipanayis A, Halaban R, Hanf B, Haussler D, Haydu LE, Hayes DN, Hayward NK, Heiman DI, Herbert L, Herman JG, Hersey P, Hoadley KA, Hodis E, Holt RA, Hoon DS, Hoppough S, Hoyle AP, Huang FW, Huang M, Huang S, Hutter CM, Ibbs M, Iype L, Jacobsen A, Jakrot V, Janning A, Jeck WR, Jefferys SR, Jensen MA, Jones CD, Jones SJM, Ju Z, Kakavand H, Kang H, Kefford RF, Khuri FR, Kim J, Kirkwood JM, Klode J, Korkut A, Korski K, Krauthammer M, Kucherlapati R, Kwong LN, Kycler W, Ladanyi M, Lai PH, Laird PW, Lander E, Lawrence MS, Lazar AJ, Łaźniak R, Lee D, Lee JE, Lee J, Lee K, Lee S, Lee W, Leporowska E, Leraas KM, Li HI, Lichtenberg TM, Lichtenstein L, Lin P, Ling S, Liu J, Liu O, Liu W, Long G V, Lu Y, Ma S, Ma Y, Mackiewicz A, Mahadeshwar HS, Malke J, Mallery D, Manikhas GM, Mann GJ, Marra MA, Matejka B, Mayo M, Mehrabi S, Meng S, Meyerson M, Mieczkowski PA, Miller JP, Miller ML, Mills GB, Moiseenko F, Moore RA, Morris S, Morrison C, Morton D, Moschos S, Mose LE, Muller FL, Mungall AJ, Murawa D, Murawa P, Murray BA, Nezi L, Ng S, Nicholson D, Noble MS, Osunkoya A, Owonikoko TK, Ozenberger BA, Pagani E, Paklina O V, Pantazi A, Parfenov M, Parfitt J, Park PJ, Park WY, Parker JS, Passarelli F, Penny R, Perou CM, Pihl TD, Potapova O, Prieto VG, Protopopov A, Quinn MJ, Radenbaugh A, Rai K, Ramalingam SS, Raman AT, Ramirez NC, Ramirez R, Rao U, Rathmell WK, Ren X, Reynolds SM, Roach J, Robertson AG, Ross MI, Roszik J, Russo G, Saksena G, Saller C, Samuels Y, Sander C, Sander C, Sandusky G, Santoso N, Saul M, Saw RP, Schadendorf D, Schein JE, Schultz N, Schumacher SE, Schwallier C, Scolyer RA, Seidman J, Sekhar PC, Sekhon HS, Senbabaoglu Y, Seth S, Shannon KF, Sharpe S, Sharpless NE, Shaw KRM, Shelton C, Shelton T, Shen R, Sheth M, Shi Y, Shiau CJ, Shmulevich I, Sica GL, Simons J V, Sinha R, Sipahimalani P, Sofia HJ, Soloway MG, Song X, Sougnez C, Spillane AJ, Spychała A, Stretch JR, Stuart J, Suchorska WM, Sucker A, Sumer SO, Sun Y, Synott M, Tabak B, Tabler TR, Tam A, Tan D, Tang J, Tarnuzzer R, Tarvin K, Tatka H, Taylor BS, Teresiak M, Thiessen N, Thompson JF, Thorne L, Thorsson V, Trent JM, Triche TJ, Tsai KY, Tsou P, Van Den BD, Van AE, Veluvolu U, Verhaak RG, Voet D, Voronina O, Walter V, Walton JS, Wan Y, Wang Y, Wang Z, Waring S, Watson IR, Weinhold N, Weinstein JN, Weisenberger DJ, White P, Wilkerson MD, Wilmott JS, Wise L, Wiznerowicz M, Woodman SE, Wu CJ, Wu CC, Wu J, Wu Y, Xi R, Xu AW, Yang D, Yang L, Yang L, Zack TI, Zenklusen JC, Zhang H, Zhang J, Zhang W, Zhao X, Zhu J, Zhu K, Zimmer L, Zmuda E, Zou L. Genomic classification of cutaneous melanoma. Cell 2015;161:1681–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Planchard D, Besse B, Groen HJM, Souquet PJ, Quoix E, Baik CS, Barlesi F, Kim TM, Mazieres J, Novello S, Rigas JR, Upalawanna A, D’Amelio AM, Zhang P, Mookerjee B, Johnson BE. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Corcoran RB, Andre T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, Hollebecque A, McRee AJ, Siena S, Middleton G, Muro K, Gordon MS, Tabernero J, Yaeger R, O’dwyer PJ, Humblet Y, de Vos F, Jung AS, Brase JC, Jaeger S, Bettinger S, Mookerjee B, Rangwala F, van Cutsem E. Research article combined BRAF, EGFR, and MEK inhibition in patients with BRAF V600E -mutant colorectal cancer. Cancer Discov 2018;8:428–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Solit DB, Rosen N. Resistance to BRAF inhibition in melanomas. N Engl J Med 2011;364:772–774. [DOI] [PubMed] [Google Scholar]
- 247. Mincu RI, Mahabadi AA, Michel L, Mrotzek SM, Schadendorf D, Rassaf T, Totzeck M. Cardiovascular adverse events associated with BRAF and MEK inhibitors: a systematic review and meta-analysis. JAMA Netw Open 2019;2:e198890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Roberts PJ, Der CJ. Targeting the RAF-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007;26:3291–3310. [DOI] [PubMed] [Google Scholar]
- 249. Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev 2010;90:1507–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, Voisin L, Saba-El-Leil MK, Meloche S, Pouysségur J, Pagès G, De Windt LJ, Doevendans PA, Molkentin JD. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation 2004;109:1938–1941. [DOI] [PubMed] [Google Scholar]
- 251. Banks M, Crowell K, Proctor A, Jensen BC. Cardiovascular effects of the MEK inhibitor, trametinib: a case report, literature review, and consideration of mechanism. Cardiovasc Toxicol 2017;17:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Glen C, Tan YY, Waterston A, Evans TRJ, Jones RJ, Petrie MC, Lang NN. Mechanistic and clinical overview cardiovascular toxicity of BRAF and MEK inhibitors: JACC: CardioOncology state-of-the-art review. JACC CardioOncol 2022;4:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Peters JM. Proteasomes: protein degradation machines of the cell. Trends Biochem Sci 1994;19:377–382. [DOI] [PubMed] [Google Scholar]
- 254. Kortuem KM, Stewart AK. Carfilzomib. Blood 2013;121:893–897. [DOI] [PubMed] [Google Scholar]
- 255. Xiao Y, Yin J, Wei J, Shang Z. Incidence and risk of cardiotoxicity associated with bortezomib in the treatment of cancer: a systematic review and meta-analysis. PLoS One 2014;9:e87671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Waxman AJ, Clasen S, Hwang WT, Garfall A, Vogl DT, Carver J, O’Quinn R, Cohen AD, Stadtmauer EA, Ky B, Weiss BM. Carfilzomib-associated cardiovascular adverse events a systematic review and meta-analysis. JAMA Oncol 2018;4:e174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Willis MS, Patterson C. Proteotoxicity and cardiac dysfunction—Alzheimer’s disease of the heart? N Engl J Med 2013;368:455–464. [DOI] [PubMed] [Google Scholar]
- 258. Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR, Day SM. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation 2010;121:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Hasinoff BB, Patel D, Wu X. Molecular mechanisms of the cardiotoxicity of the proteasomal-targeted drugs bortezomib and carfilzomib. Cardiovasc Toxicol 2017;17:237–250. [DOI] [PubMed] [Google Scholar]
- 260. Ranek MJ, Zheng H, Huang W, Kumarapeli AR, Li J, Liu J, Wang X. Genetically induced moderate inhibition of 20S proteasomes in cardiomyocytes facilitates heart failure in mice during systolic overload. J Mol Cell Cardiol 2015;85:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Fomin A, Gärtner A, Cyganek L, Tiburcy M, Tuleta I, Wellers L, Folsche L, Hobbach AJ, von Frieling-Salewsky M, Unger A, Hucke A, Koser F, Kassner A, Sielemann K, Streckfuß-Bömeke K, Hasenfuss G, Goedel A, Laugwitz KL, Moretti A, Gummert JF, dos Remedios CG, Reinecke H, Knöll R, van Heesch S, Hubner N, Zimmermann WH, Milting H, Linke WA. Truncated titin proteins and titin haploinsufficiency are targets for functional recovery in human cardiomyopathy due to TTN mutations. Sci Transl Med 2021;13. [DOI] [PubMed] [Google Scholar]