Figure 1.
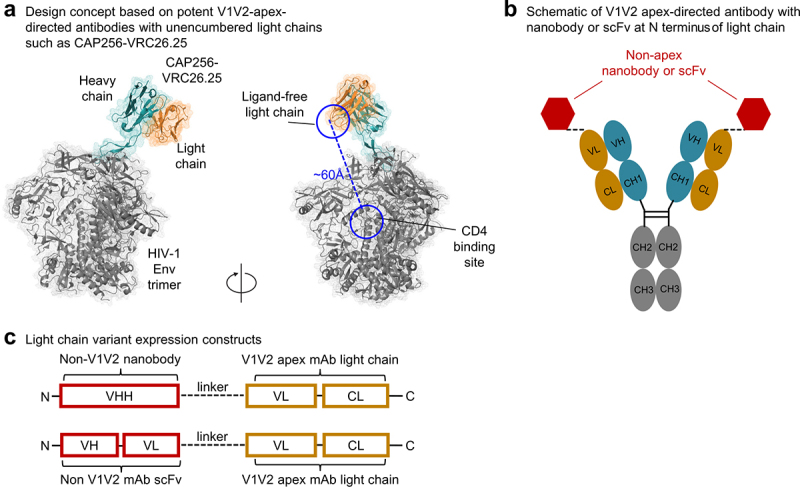
Design of HIV-1 bispecific antibodies attaching nanobodies or scFvs to the light chain of the super potent V2-apex-directed antibody CAP256-VRC26.25. (a) Structure of the antigen-binding fragment (Fab) of antibody CAP256-VRC26.25 in complex with a prefusion-closed HIV-1 Env trimer showing an unencumbered light chain allowing its linkage to other HIV-1 trimer-binding components. The resultant bispecific antibody enables synergistic binding and enhanced neutralization. (b) Schematic of V1V2 antibody with additional binding component genetically fused to the light chain N terminus. (c) Light chain variant expression constructs. Sequence information of these variants are listed in supplementary figures. Upper construct shows light chain variants linked with nanobody. Bottom construct shows light chain variants linked to single-chain Fv.
