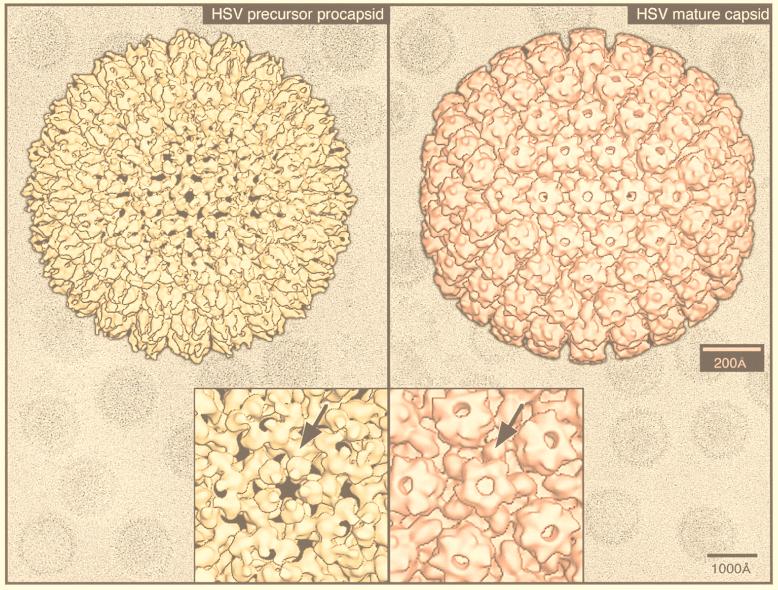
FIG. 34.
Procapsid maturation in HSV-1 (311, 312). Shown are isosurface views along a twofold direction of cryoreconstructions of the T=16 HSV-1 precursor procapsid at 28-Å resolution (left), mature capsid at 25-Å resolution (right), close-up views of each capsid along a fivefold axis (insets). The color images are overlaid on the unprocessed cryo-EM images of the corresponding vitrified specimens. Comparison of the two structures reveals the substantial changes that occur in the hexons (hexameric capsomers), which are distorted and nonsymmetric in the procapsid but become symmetric in the mature capsid. HSV-1 capsids contain three major proteins: VP5 (150 kDa; 960 copies), VP19c (50 kDa; ∼320 copies), and VP23 (34 kDa; ∼640 copies). The 320 “triplexes” (arrows in insets), which occupy local threefold axes of symmetry, consist of two copies each of VP23 and one copy of VP19c. The horns of density at the tips of the hexons, but not pentons, in the mature capsids are monomers of VP26 (12 kDa), an additional viral protein that is dispensable for capsid assembly but which binds to the capsid if present. Adapted from reference 294 with the permission of the author and the publisher.