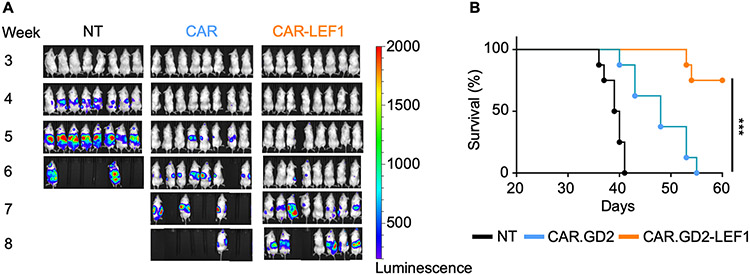
Figure 6: CAR-LEF1 NKTs have superior in vivo therapeutic activity versus parental CAR.GD2 NKTs.
A, NKTs were expanded with IL2 and transduced with either CAR.GD2 (CAR) or CAR.GD2-LEF1 (CAR-LEF1), with a non-transduced (NT) control group. NSG mice (n = 8 mice per group) were i.v. injected with 1 x 106 luciferase-transduced CHLA-255 cells on day 0. On day 7, mice received an i.v. injection of NT, CAR, or CAR-LEF1 NKT preparations (4 x 106 CAR+ cells per mouse). IL2 was injected i.p. three times a week for two weeks after NKT injection. Tumor growth was monitored using bioluminescence imaging once per week. One experiment was performed. B, A survival plot was generated using the Kaplan–Meier method. Differences in survival probability were compared using the log-rank test. ***P < 0.001.