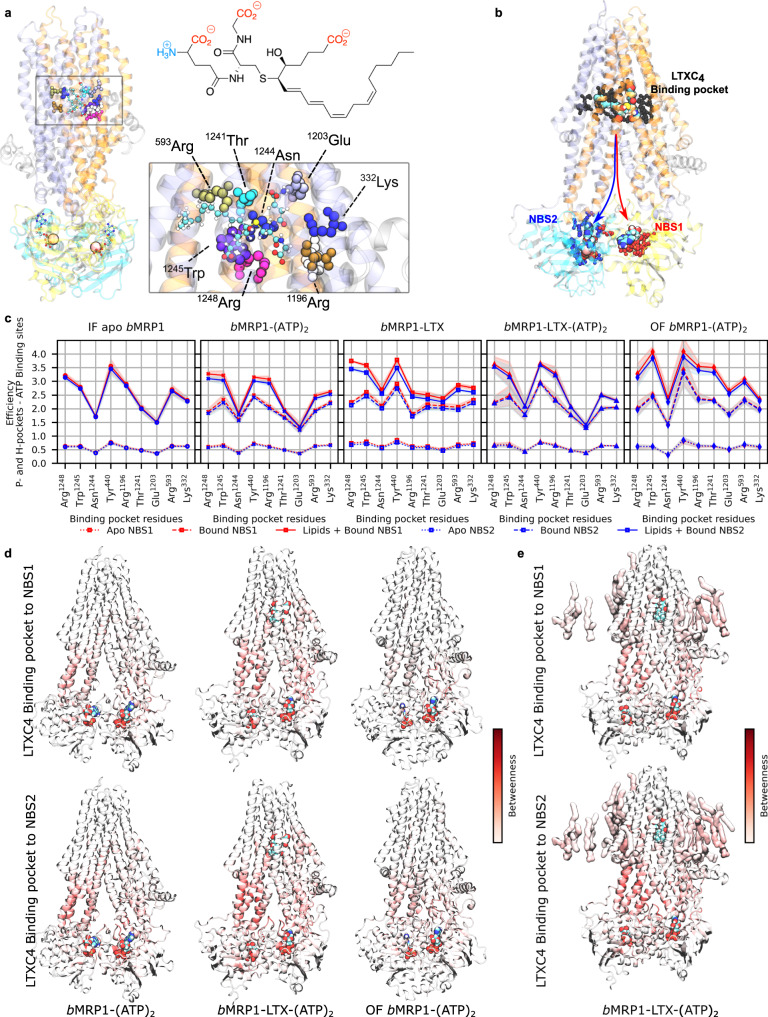
Fig. 3. Substrate-bMRP1 interactions and subsequent allosteric communications to nucleotide-binding sites.
a Substrate-binding pocket highlights important residues to leukotriene C4 binding. The structure of leukotriene is also shown for which amphiphilic features are stressed out. b Definition of the allosteric pathway investigated in the present study for which NBS1 and NBS2 were treated separately. c Calculated allosteric efficiencies of the information flow between substrate-binding pocket and NBS1 (red) or NBS2 (blue) for the different systems embedded in POPC:POPE:Chol (2:1:1). Solid, dashed and dotted lines respectively depict efficiencies considering: Protein + lipids + nucleotides/substrate, Protein + nucleotides/substrate and standalone Protein. Standard errors are shown as shades and were calculated from n = 3 replicas treated independently for each system. d Protein and e lipid contributions to the information flow for allosteric communication from substrate-binding pocket to NBS1 and NBS2 show that NBD2 and its coupling helices (CH4-5 and CH8-9) are systematically involved regardless of the sink region.