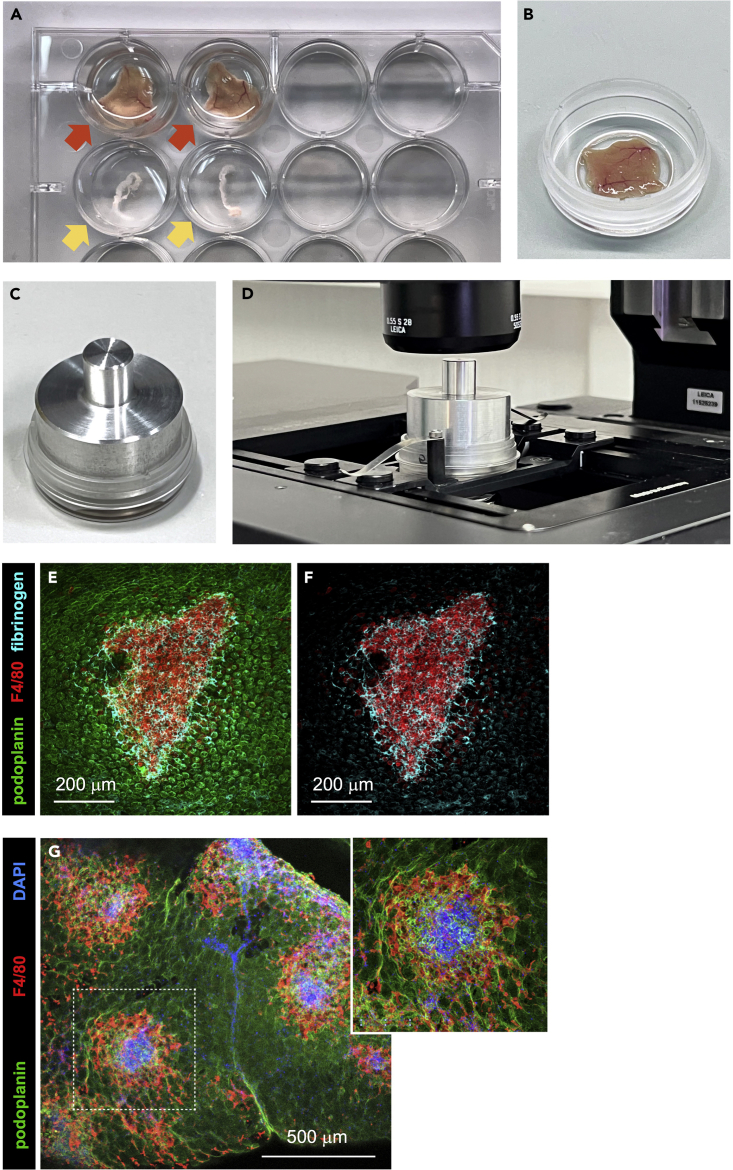
Figure 2.
Immunofluorescence staining of whole peritoneal wall and omentum samples
(A) Peritoneal wall (red arrows) and omentum (yellow arrows) samples transferred to wells of a 12-well plate to carry out the immunofluorescence staining process.
(B) Peritoneal wall sample transferred to a μ-Dish 35 mm dish with No. 1.5 ibidi Polymer Coverslip containing 300 μL of mowiol-based WMI mounting solution.
(C) Peritoneal wall sample mounted in a μ-Dish 35 mm dish with No. 1.5 ibidi Polymer Coverslip, with 300 μL of mowiol-based WMI mounting solution, and a peritoneal wall aluminum weight on top.
(D) Peritoneal wall sample mounted in a μ-Dish 35 mm dish with No. 1.5 ibidi Polymer Coverslip with aluminum weight on top, placed on the stage of a confocal microscope.
(E and F) WMI/confocal microscopy imaging of a resMØ-aggregate in the peritoneal wall, at 4 h after E. coli infection, after immunofluorescent staining with Alexa Fluor 488-conjugated anti-podoplanin (mesothelium), Alexa Fluor 594-conjugated anti-F4/80 (resMØs), and unconjugated anti-fibrinogen (fibrin network) followed by Alexa Fluor 647-conjugated secondary antibody. (E) podoplanin, F4/80 and fibrinogen staining; (F) F4/80 and fibrinogen staining. Images were acquired on a multispectral Leica TCS SP8 confocal microscope and analyzed using ImageJ software.
(G) Low magnificaction confocal microscopy image of the omentum, at 4 h after E. coli infection, showing the distribution of resMØ-aggregates after immunofluorescent staining with Alexa Fluor 488-conjugated anti-podoplanin (mesothelium), Alexa Fluor 594-conjugated anti-F4/80 (resMØs), and DAPI (nuclei). Insert: enlargement of the area marked in G with a white dotted line. Images were acquired on a multispectral Leica SP8 confocal microscope and analyzed using ImageJ software. Figures F and G reprinted with permission from Vega-Pérez at al.1