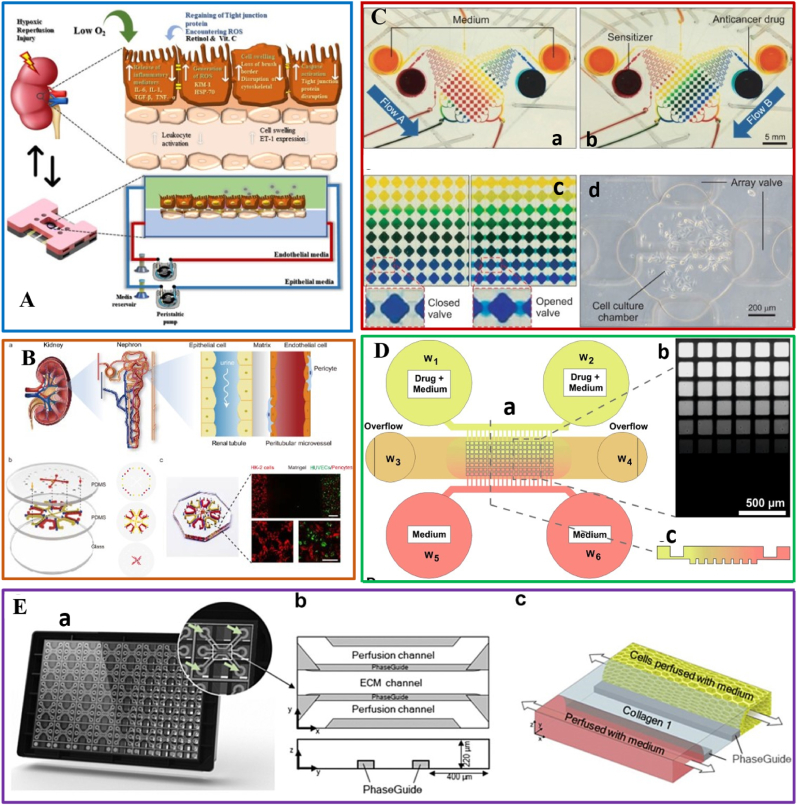
Fig. 6.
Disease and high-throughput drug screening-on-chips in urinary system. (A) Schematic of renal hypoxia-reperfusion injury-on-chip model [102]. Reproduced with permission [102]. Copyright 2022, American Chemical Society; (B) Construction of an integrated functional tubule-vascular microfluidic chip. (a) An illustration of the characteristic physiological structures of the renal interstitial microenvironment. (b) Schematic image of a microfluidic renal interstitium-on-a-chip device with microchannels replicating the renal tubules and peritubular microvessels. (c) Photograph of the microfluidic chip (left), and the representative image of the co-culture of HK-2 cells, HUVECs, and pericytes in the chip (right) [103]. Reproduced with permission [103]. Copyright 2022, Elsevier BV; (C) Demonstration of concentration gradient in microfl uidic system using color dye solution (a) Trapping of 8 different concentration gradient of a sensitizer through a diffusive mixer by driven micropump from two reservoirs (sensitizer and medium are represented by red and yellow respectively) (b) The second trapping of 8 different concentration gradient of drug (drug was represented by blue color dye) (c) Concentration gradient of color dye was maintained 4 h after closing valves without perfusion of reagent (d) Representative cell culture chamber with PC3 cells trapped and grown for 24 h [118]. Reproduced under terms of the CC-BY license [118]. Copyright 2014, Korean Society of Applied Pharmacology; (D) Microfluidic device layout and functions. (a) Schematic representation of the device structure. (b) Fluorescent image showing a calcein concentration gradient over the micro-well array. (c) Schematic cross-section of the gradient generated along a column of the spheroid array [119]. Reproduced under terms of the CC-BY license [119]. Copyright 2018, Springer Nature; (E) The human proximal renal tubule-on-chip for the study of nephrotoxicity and drug interaction. (a) Image of the back side of the OrganoPlate 3-lane. The microfluid network is positioned in-between a glass sandwich of two microscope grade glass plates which are attached to the bottom of a standard 384 titer well plate. (b) Schematic of one chip presenting two perfusion channels and the extracellular matrix (ECM) channel in the middle. (c) Artist impression of one chip. The chip was loaded with collagen 1 (blue) to the ECM channel and proximal tubule cells (yellow) were seeded to the top channel [117]. Reproduced under terms of the CC-BY license [117]. Copyright 2021, Elsevier.