Figure 1. Procedure of Including Study Participants.
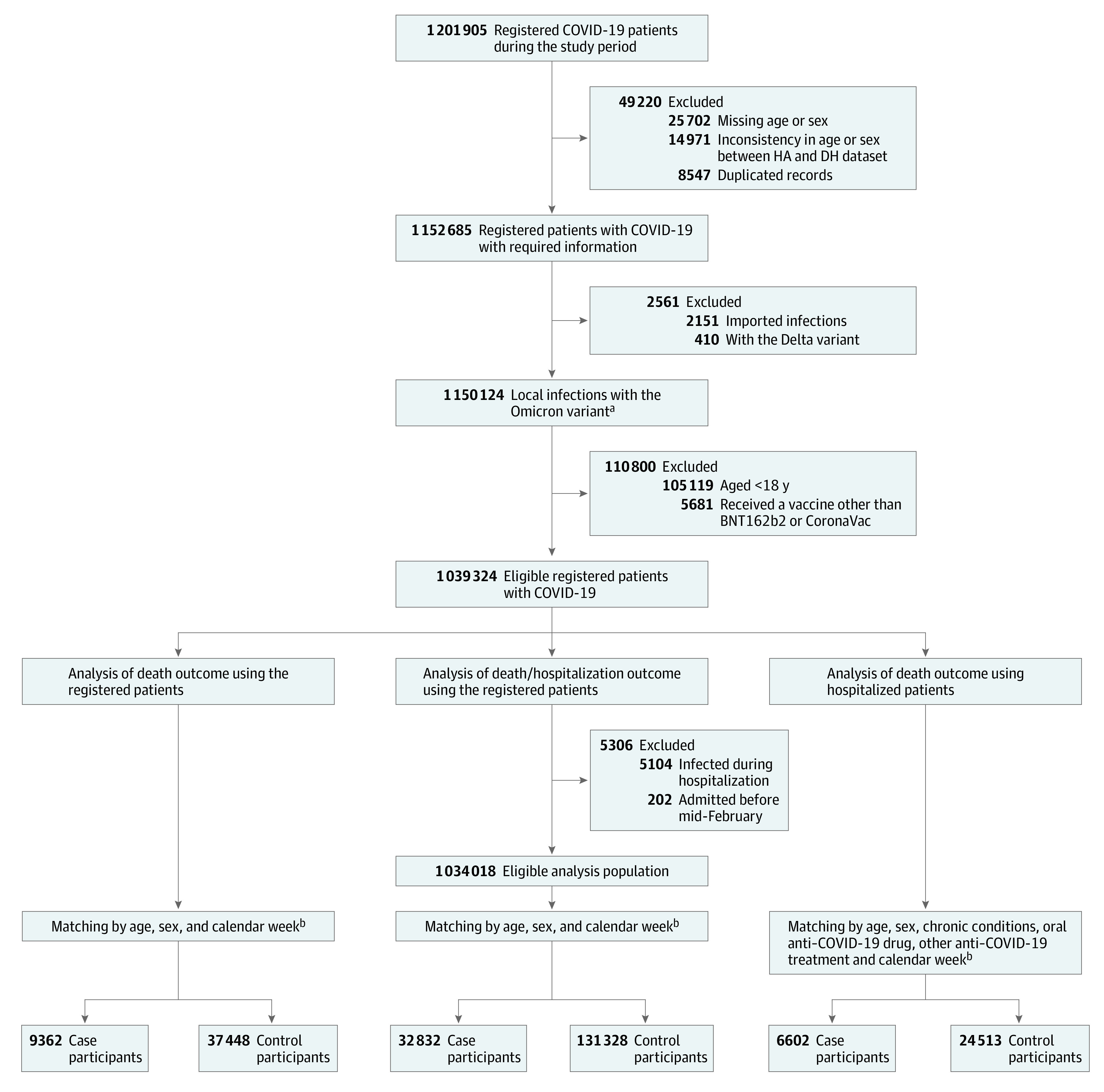
DH indicates Department of Health; HA, Hospital Authority.
aThe Omicron variant status of patients with polymerase chain reaction–confirmed COVID-19 was determined by genomic sequencing based on the defining mutations (N501Y and E484A) before mid-February 2022. Due to laboratory capacity being overwhelmed by the rapid surge in cases, all confirmed cases using either polymerase chain reaction testing or rapid antigen tests were included after mid-February 2022, a period predominated by the SARS-CoV-2 Omicron variant.
bThe propensity score method was conducted for all case-control matches in a 1:4 ratio, and the scores were determined based on the available covariates.
