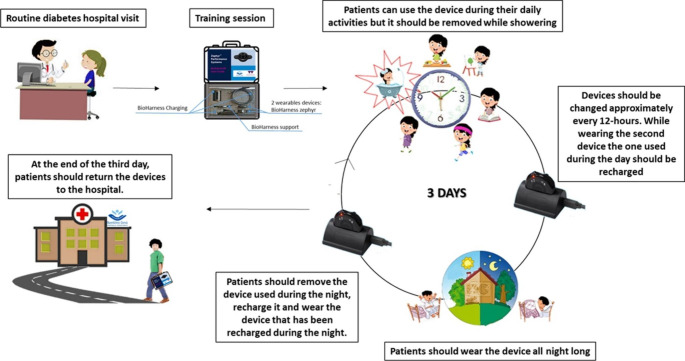
Fig. 1.
Shows the study procedure. During the patients’ routine diabetes hospital visit, recruited patients (that already use CGM sensors) are asked to wear an additional wearable device, Medtronic Zephyr BioPatch, for recording the physiological data for a period of up to 3 days. After receiving the training session and relevant information about the study, the participants are allowed to return home with the wearable device attached. During the hospital visit, the PEdsQL is submitted to recruited patients. During the monitoring days, patients can continue their daily activities undisturbed, without any changes in either physical activities or diet. They should wear the sensor during the day and the night and remove it while showering. The device should be approximately charged every 12-hours. For this reason, patients were provided with two devices. While wearing the second device the one used during the day should be recharged and vice versa. At the end of the third day, patients should return the devices to the hospital (This picture was created by the authors)