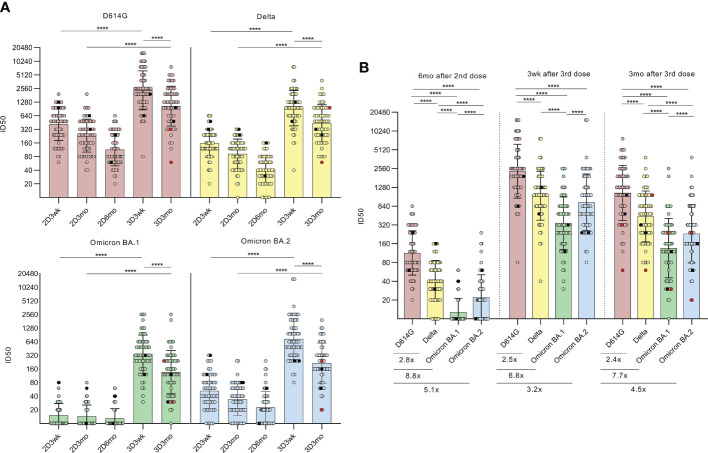
Figure 2.
Neutralizing antibodies after the second and third COVID-19 mRNA vaccine doses. (A), Microneutralization test was used to analyze the neutralizing antibody responses of HCWs (n=64) against D614G strain and variants Delta, BA.1, BA.2 after the second vaccine dose at three-week (2D3wk, n=60, BA.2 n=59), three-month (2D3mo, n=60, BA.2 n=59) and six-month (2D6mo, n=62, BA.2 n=61) time points as well as after the third vaccine dose at three-week (3D3wk, n=63, BA.2 n=62) and three-month (3D3mo, n=59, BA.2 n=58) time points. HCWs with PCR-confirmed SARS-CoV-2 infection prior to the first vaccination (n=2) are marked with black dots and breakthrough infections after the third dose (n=2) are marked with red dots. Differences between time points (2D3wk vs 3D3wk; 2D3mo vs 3D3mo; 3D3wk vs 3D3mo) were analyzed with Wilcoxon signed-rank test. (B), Neutralization titers between D614G strain and variants Delta, BA.1, and BA.2 at six months after the second vaccine dose as well as three weeks and three months after the third vaccine dose were compared and are represented as fold differences below the figures. Wilcoxon signed-rank test was used to analyze the statistical significance, and two-tailed p-values <0.05 were considered statistically significant. Samples with no data on both data points were excluded from the analyses. The geometric means with geometric SDs are shown in the figures. ****p<0.0001.