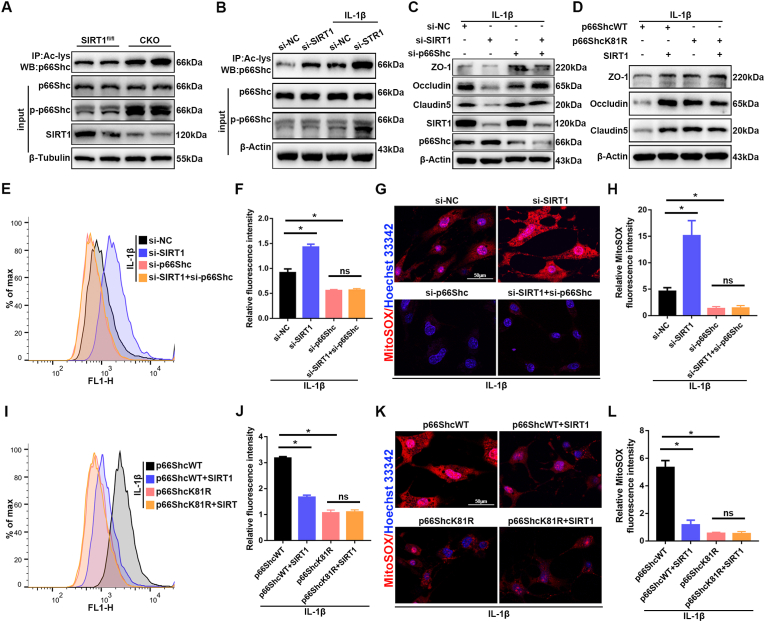
Fig. 8.
Endothelial SIRT1 attenuates oxidative stress and endothelial disruption via p66Shc. (A) The acetylation levels of p66Shc were assessed in lysates from spinal cord tissues of SIRT1fl/fl and SIRT1 CKO mice at 3 d after SCI by immunoprecipitation with anti-acetyl-lysine antibody followed by immunoblotting with anti-p66Shc antibody. The phosphorylation levels of p66Shc were determined by immunoblotting with anti-p-p66Shc antibody (n = 6 animals per group). The acetylation and phosphorylation levels of p66Shc were higher in SIRT1 CKO mice than SIRT1fl/fl mice at 3 d after SCI. (B) bEnd.3 cells from different groups (si-NC versus si-SIRT1) were treated with or without 10 ng/ml IL-1β for 24 h. Western blotting analysis was used to detect changes in the acetylation and phosphorylation levels of p66Shc (n = 3 samples per group). Knockdown of SIRT1 increased the acetylation and phosphorylation levels of p66Shc. (C) bEnd.3 cells were transfected with si-NC or si-SIRT1 or si-p66Shc, then treated with 10 ng/ml IL-1β for 24 h. Western blotting analysis was performed to detect the expression of TJ proteins (n = 3 samples per group). (D) bEnd.3 cells were transfected with p66ShcWT or p66ShcK81R or SIRT1 plasmids, then treated with 10 ng/ml IL-1β for 24 h. Western blotting analysis was performed to detect the expression of TJ proteins (n = 3 samples per group). (E, F, I, J) Detection and quantification of ROS levels in the bEnd.3 cells in the indicated groups by flow cytometry (n = 3 samples per group). (G, H, M, N) Immunofluorescence detection and quantification of MitoSOX in bEnd.3 cells in the indicated groups (n = 3 samples per group). *P < 0.05; ns, not significant.