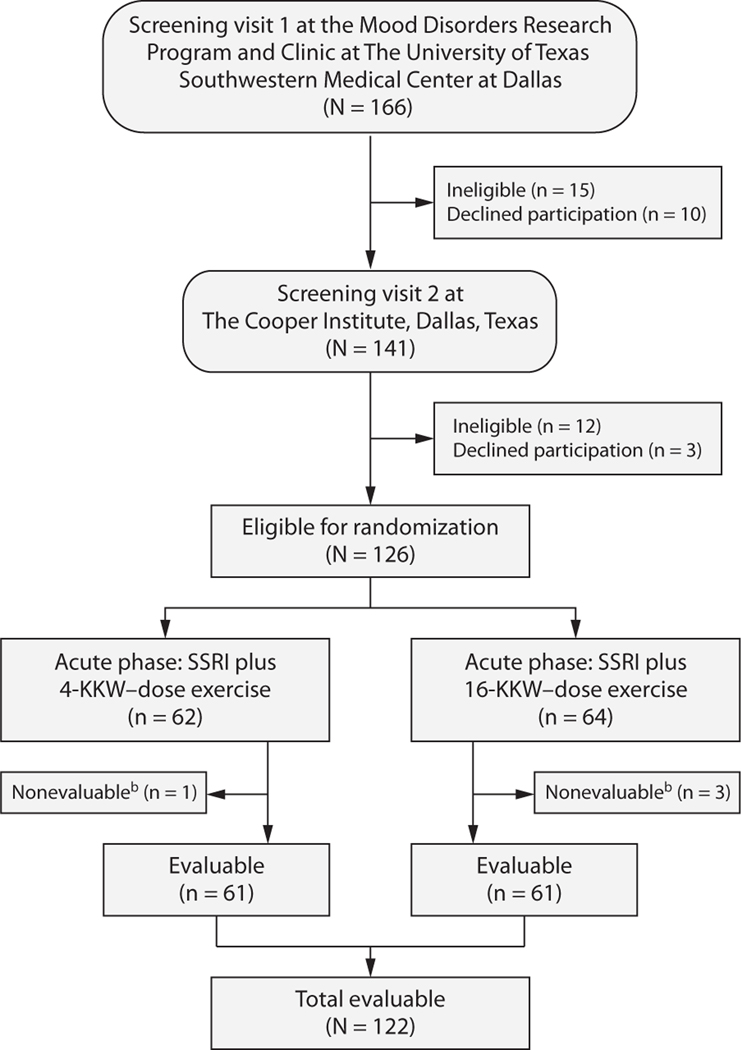
Figure 1. Flow Diagram of Participants in the TReatment with Exercise Augmentation for Depression (TREAD) Studya.
aNote that 25 participants exited the study before completing the full 12 weeks of the acute phase for the following reasons: 2 for adverse events (both in the 16-KKW group), 10 due to inconvenience or time constraints (2 in the 4-KKW group, 8 in the 16-KKW group), 5 because they had worsening symptoms and/or desired or required a different treatment (3 in the 4-KKW group, 2 in the 16-KKW group), 7 due to noncompliance (3 in the 4-KKW group, 4 in the 16-KKW group), and 1 who was lost to follow-up (in the 4-KKW group). However, all of these participants, with the exception of the 4 nonevaluable participants who had no postbaseline data, are included in the analyses.
bNo postbaseline data available.
Abbreviation: SSRI = selective serotonin reuptake inhibitor.