Figure 2.
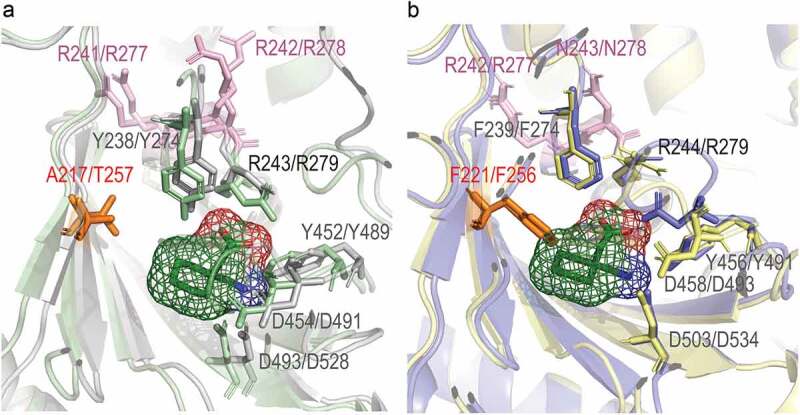
Gabapentinoid and amino acid–binding pockets of α2δ-1 – α2δ-4. Phe221/Phe256 (F221/F256) creates steric hindrance in the ligand-binding pocket of α2δ-3 and α2δ-4 proteins. Superimposed structures of α2δ-1 and α2δ-2 (a) and α2δ-3 and α2δ-4 (b) ligand-binding pockets. α2δ-1 is the rabbit protein cryo-EM structure, α2δ-2 to α2δ-4 are the AlphaFold models. Gabapentin is docked to the binding pocket of α2δ-1. Each of α2δ-2 to α2δ-4 was superimposed on α2δ1; α2δ-3 and α2δ-4 then were extracted and placed on panel B for clarity. A figure with all the proteins simultaneously superimposed on α2δ-1 can be found on GitHub at the following link https://github.com/ToshkaDev/Alpha2Delta-proteins-review. In pink font the first two residues of the RRR (in α2δ-1 and α2δ-2)/RNR (in α2δ-3 and α2δ-4) sequence are shown – as can be seen they are not involved in ligand binding. Other residues, except for the residue corresponding to Asp454 (D454) in α2δ-1, denote the amino acid–binding motif .[35]
