Abstract
Central nervous system infection by Balamuthia mandrillaris is a rare and severe condition, which has a fatality rate of approximately 95% and often evades timely diagnosis due to its rarity and non-specific clinical manifestations. Here, we report a case of encephalomyelomeningitis caused by B. mandrillaris in a male who presented with transient coma, nausea, and vomiting when working in a garbage dump. Initial magnetic resonance imaging (MRI) of the brain showed normal signals. Despite receiving steroids as well as antibacterial and antiviral treatment, he developed urinary and fecal dysfunction, inability to walk, and deterioration of consciousness. Both brain and spinal cord MRI revealed abnormal findings, and next-generation sequencing of the cerebrospinal fluid showed the presence of B. mandrillaris. A combination of fluconazole and albendazole was administered; however, the patient deteriorated gradually and died 30 days after the onset. We suggest the unbiased metagenomic sequencing of the affected tissues/CSF in patients with CNS infections that are difficult to diagnose or treat, and multiple tests at different stages of the disease may be required.
Keywords: Balamuthia mandrillaris amoeba, encephalomyelomeningitis, cerebrospinal fluid, next-generation sequencing, case report
Introduction
Balamuthia mandrillaris is a pathogenic free-living amoeba that infects mainly the skin and central nervous system (CNS) and may present with skin lesions, granulomatous amoebic encephalitis (GAE), and primary meningoencephalitis (PAM), which was first reported in humans in 1990 and more than 200 cases have been diagnosed worldwide.1 Majority of cases were reported from warmer parts of the globe, particularly Latin America and the southwestern United States, and few cases were reported from Europe and Asia-Pacific. B. mandrillaris has two life cycle stages: trophozoites (representing the infective stage) and cysts, it is pervasive in freshwater, soil, dust, sewage, swimming pools, humidifiers, and water storage tanks, through either the contaminated broken skin directly or inhalation of the cysts via the mouth or nose.2 B. mandrillaris can infect the body via the nasal/olfactory nerve, lung and gastrointestinal tract, invade the CNS through hematogenous dissemination and cause B. mandrillaris meningoencephalitis (BAE). Notably, organ transplants are also a means of B. mandrillaris invasion. Clinical presentation of BAE is similar to those of viral or bacterial meningitis and nonspecific in neuroimaging and infrequently detected in common microbiology tests for cerebrospinal fluid (CSF).3 The low morbidity, difficulty of diagnosis, and lack of proven effective treatments make it a fatality rate of about 95%, for only about 10/200 patients with BAE who have survived CNS invasion.4 Despite this, some promising studies conjugating existing drugs with nanoparticles and other novel therapies have been reported.5 Here, we report a case of B. mandrillaris encephalomyelomeningitis in a patient and review the literature.
Case Report
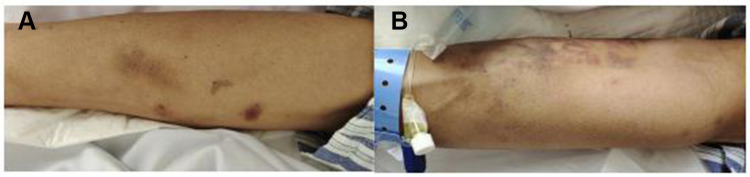
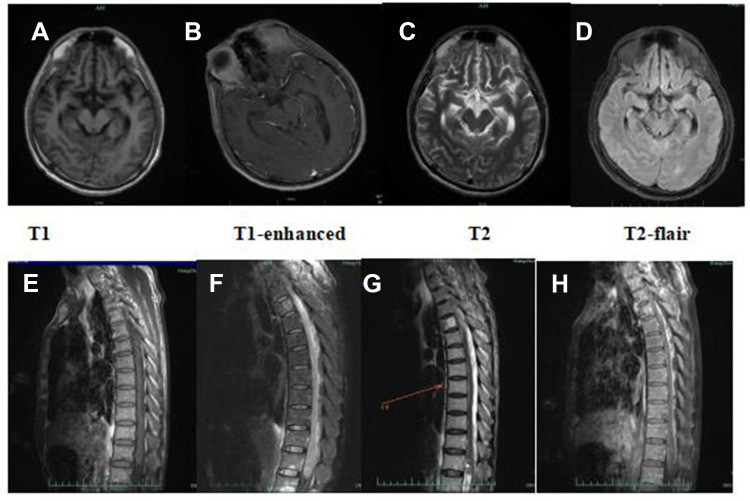
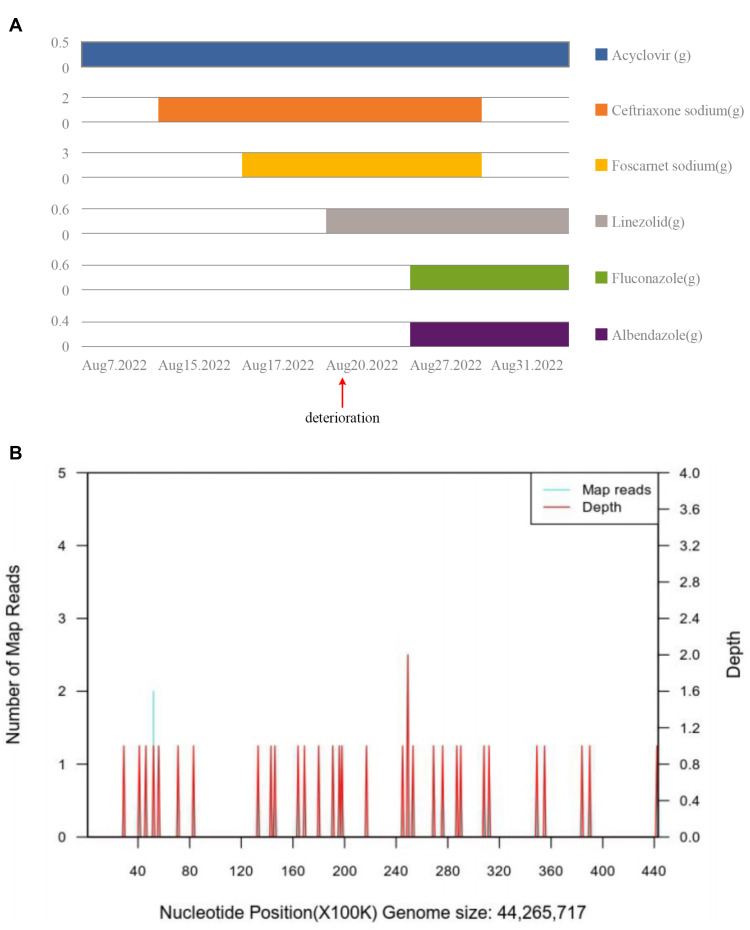
A 56-year-old man who had been in good health presented with transient coma, nausea and vomiting when working, then visited the local hospital. MRI showed no visible abnormalities in the brain, the CSF pressure on the second day of the illness was 275mmH2O, karyocytes, 5/μL; protein, 3.584 g/L; glucose, 6.92 mmol/L; and chloride, 105.7 mmol/L. However, the patient developed drowsiness and severe headache despite receiving symptomatic treatment. Four days later, he was admitted to the neurology department of our hospital. A neurological examination showed hypersomnia, stiff neck, a positive kernig sign, normal muscle strength in the limbs. Other physical examinations revealed dark red lesions (about 1×1 cm and 10×5 cm) located on the right and left forearms, respectively (Figures 1A and B). He denied any recent travel, history of freshwater swimming, or any intake of undercooked meat or unpasteurized dairy products. Before his admission, he had worked in a garbage dump for about 2 years. The patient’s medical history included hypertension, and diabetes diagnosed after admission. He has no family history of genetic disorders. The CSF appears yellowish and the pressure was 250mmH2O at 1 week of illness. Results of the CSF examination are as follows: karyocytes, 8/μL; red cells, 0/μL; protein, 1.889 g/L (normal range, 0.150–0.450 g/L); glucose, 7.6 mmol/L (normal range, 2.5–4.5 mmol/L); and chloride, 110 mmol/L (normal range, 120–132 mmol/L). IgG (16.50g/L, normal range, 0.50–5.0 g/L) in the CSF significantly increased, with the positive of varicella-zoster virus (VZV) IgG, herpes simplex virus (HSV) I IgG and cytomegalovirus (CMV) IgG. No abnormalities were seen in NGS. India ink staining, acid-fast stain, bacterial and bacterial and fungal cultures, and cytology of CSF were all negative. Routine laboratory values, including an erythrocyte sedimentation rate, procalcitonin and C-reactive protein, were within normal limits. The complete blood count was abnormal (90.3% neutrophilic granulocytes, 4.9% lymphocytes and 0% eosinophil). His lymphocyte count and immune cell subpopulations were reduced (CD3T lymphocytes, cytotoxic T cell CD8, helper T-cells CD4 and natural killer cell CD16/56). Brain MRI showed lesions enhanced, including the bilateral perimesencephalic cistern, sylvian fissure and cerebral longitudinal fissure and its peripheral cerebral cortex, as well as the frontal lobe and parietal lobe (Figure 2A–D). The diagnosis of CNS infection led to combined therapy with acyclovir (500 mg, BID, ivgtt), ceftriaxone sodium (2 g, QD, ivgtt), foscarnet sodium (3g, Q12H), and dexamethasone (10 mg, QD) (Figure 3A).
Figure 1.
Skin lesions presented with a 1×1 cm dark red a round-like on the right upper arm (A) and a 10×5 cm irregularly shaped dark red on the left inner forearm (B), without protruding from the skin surface, without pruritus, pain or desquamation.
Figure 2.
(A–D) Brain MRI on day 5 of onset revealed multiple enhanced lesions on the bilateral perimesencephalic cistern, sylvian fissure and cerebral longitudinal fissure and its peripheral cerebral cortex, as well as the frontal lobe and parietal lobe; (E–H) MRI of the thoracic segment on day 14 of onset revealed the 2–4, 12 thoracic medullary enlargements, with longer T1, T2 signals, and spinal cord membrane thickening with cystic image, which were enhanced.
Figure 3.
(A) Graphical timeline of drug administration. (B) Results of next-generation sequencing in cerebrospinal fluid, B. mandrillaris genome coverage map.
His condition continued to worsen on day 14 of onset, he developed lethargy, urinary and fecal dysfunction as well as weakness of both lower limbs, neurological examination showed the muscle strength of both lower limbs (2/5). MRI of the thoracic segment revealed that the spinal cord with patchy longer T2 signals, which were vaguely enhanced (Figure 2E–H). We do not suppose that the patient deterioration due to the toxicity of many drugs applied during the treatment. The patient had a 5kg weight loss and two fevers since admission, with a temperature of 37.6°C and 37.8°C. We suspect the possibility of tuberculous meningoencephalitis and are pending a second NGS result. After consultation with infectious disease specialists, administered linezolid (600 mg, Q12H) was to treat the suspected infection, with no progressive worsening of neurological symptoms temporally since then. Mild change in CSF on day 15 post-onset compared to the previous (Table 1), fortunately, a second NGS identified B. mandrillaris (Figures 3B). He was diagnosed with encephalomyelomeningitis caused by B. mandrillaris and then treated with albendazole (400 mg, QD, po) and fluconazole (600 mg, QD, ivgtt). On day 18 of the onset, he had frequent bleeding stools despite receiving enhanced gastric mucosal protection therapy, the anorectal surgeon would not consider hemorrhoids after a digital rectal examination and suggested further gastrointestinal endoscopy to exclude gastrointestinal tumor, the man’s family refused and request a transition home, he died 1 week after discharge.
Table 1.
A Summary and Comparison of CSF Characteristics at Different Stages of the Disease
| CSF Features | Initial Pressure (mmH2O) | Karyocytes (/μL) | Sugar Level (mmol/L) | Chloride (mmol/L) | Protein (g/L) | Others |
|---|---|---|---|---|---|---|
| 2 day | 275 | 5 | 6.92 | 105.7 | 3.584 | – |
| 7 day | 250 | 8 | 7.60 | 110.0 | 1.889 | VZV IgG (+), HSVI IgG (+), CMV IgG (+) |
| 14 day | 400 | 0 | 7.6 | 108.0 | 1.985 | VZV IgG (+-), HSVI IgG (-) |
Discussion
Here, we report a middle-aged male patient who was diagnosed with encephalomyelomeningitis caused by B. mandrillaris infection. He was admitted with non-specific neurological symptoms, both brain and several spinal cord segments MRI showed abnormal signals, and NGS identified B. mandrillaris infection. Despite receiving a combination of various antimicrobials, his prognosis worsened gradually, and he died 30 days after the disease onset.
Although the actual incidence of BAE is unknown, BAE is relatively rare and has an extremely high mortality rate. The life cycle of B. mandrillaris is divided into two phases: trophozoites and cysts, which represent the infective phase and resting or chronic infection phase, respectively.6 B. mandrillaris exists in freshwater, soil, dust, sewage, swimming pools, humidifiers, and water storage tanks,5,7–9 it can invade the individual through contaminated broken skin directly or inhalation of the cysts via mouth or nose.10 A recent case of B. mandrillaris patients who have undergone organ transplants.11 Notably, due to global warming caused by climate change and the shortage of water globally, the public has become increasingly dependent on water tanks, which are often brimming with microorganisms including B. mandrillaris and are more common in developing countries.5 B. mandrillaris has multiple routes of infection into the body via the nasal/olfactory nerve,12 lung, and gastrointestinal tract;13 it can invade the CNS through hematogenous dissemination and cause BAE, most likely gaining access through the blood–brain barrier (BBB). Epidemiologically, soil-exposed individuals engaged in horticulture, agriculture, and ranching are the high-risk group.14 There is no evidence of seasonality in the symptom onset. BAE can develop both in immunocompetent and immunocompromised individuals (eg, with tumors, diabetes, or HIV)15,16 ; the latter may increase the risk of CNS infection or accelerate the disease progression. Studies have shown that macrophages have no inhibitory effects on the biological properties of B. mandrillaris in vitro, which also supports that B. mandrillaris may cause infection in both immunocompetent and immunocompromised individuals.17 Here, we describe a male patient who had poorly controlled diabetes and worked in a garbage dump with the possibility of inhaling pathogens. Unfortunately, we were unable to perform a pathological examination of the lesions on both forearms to clarify if this case was a cutaneous manifestation of B. mandrillaris infection.
B. mandrillaris affects two of the major organ systems in humans: the skin and CNS. According to previous reports, the condition can be divided into two types based on the clinical manifestations of the patients. One type is mainly seen in the United States, which presents with encephalitis without skin lesions, and the patients usually die within weeks.14,18 The second type is observed mainly in China and Peru,19–21 where the skin is usually involved first, and then encephalitis develops gradually. Typically, skin lesions precede CNS invasion, and the time between the appearance of skin lesions and the occurrence of neurological symptoms varies from 1 month to approximately 2 years.2 The lesions usually present as painless plaques or ulcers and are commonly seen in the center of the face but may occasionally occur on the trunk, hands, and feet.22 Initial CNS symptoms often present as headache and photophobia, followed by a range of symptoms including nausea and vomiting, fever, myalgia, weight loss, and seizures.23 Laboratory tests such as blood counts and CSF examination lack specificity, and it is difficult to detect the pathogen by CSF cultures.2 The typical neuroimaging presentation of BAE is multiple well-defined, focal, ring-enhancing, space-occupying lesions with perifocal edema; hydrocephalus and ventriculomegaly have also been described. Another pathological feature is hemorrhage into the mass lesion.24,25 Any cortical lobe can be affected in patients with BAE due to B. mandrillaris, but the temporal lobe is the most prevalent, followed by the frontal lobe. Among the extracortical sites, the thalamus, cerebellum, basal ganglia including the caudate nucleus, and brainstem are the most severely exposed sites.26 Our patient presented with encephalomyelitis with headache, impaired consciousness, bilateral lower extremity paralysis, and urinary and fecal dysfunction. No typical granulomatous lesions of the brain parenchyma were seen on multiple MRI examinations performed at different phases of the disease course, which is different from previously reported cases.
Non-specific symptoms are the major reason for diagnosis of B. mandrillaris meningoencephalitis being difficult, since they can also be seen in other CNS infections, such as tumors, viral/bacterial or tuberculosis meningoencephalitis, acute disseminated encephalomyelitis, neurocysticercosis, or toxoplasmosis. Our patient had poorly controlled diabetes that possibly made him immunocompromised. Frequent blood in the stool indicated the possible presence of a gastrointestinal tumor; however, we could not determine whether the immune deficiency was caused by a tumor since the family refused gastroscopy. The TBNK lymphocyte subpopulations of CD3 T lymphocytes, cytotoxic CD8 T cells, CD4 helper T cells, and CD16/56 natural killer cells were reduced, further supporting immune deficiency. Experimental infections in mice lacking a lymphocyte subpopulation demonstrated the importance of CD4 T cells in resistance to intranasal B. mandrillaris infection and BAE.27 Previous studies have shown that anti-N-methyl-d-aspartate (anti-NMDA) receptor encephalitis can be triggered by herpes simplex virus (HSV) infection, there is a remarkable correlation between HSV-1 infection and the development of anti-NMDAR autoimmune encephalitis (AE),28 and there is a cytokine/chemokine phase between initial HSV encephalitis and secondary AE. One possible explanation for the potential immunological link between VZV IgG (+), HSVI IgG (+), and CMV IgG (+) on CSF examination and B. mandrillaris meningoencephalitis is that the severe stress reaction caused by the primary B. mandrillaris meningoencephalomyelitis triggers immune activation in the CNS. Another possibility is that a previous viral infection disrupts the BBB, increasing the chances of neurological infection by B. mandrillaris. NGS technology can provide a rapid, early etiologic diagnosis without the need for a local brain biopsy, facilitating early treatment. Clinicians should be aware that a single negative result on CSF does not rule out the diagnosis of BAE when the diagnosis of infection is not supported by polymerase chain reaction or other test results, and multiple tests at different stages of the disease may be required.
Considering the difficulty in diagnosing the infection and the unavailability of effective antimicrobial drugs, the prognosis of most patients is poor. Although we made a clear and timely diagnosis in this case, we could not prevent the rapid deterioration of the disease. The optimal antimicrobial therapy for the treatment of B. mandrillaris infection is yet to be determined. The option of antimicrobial agents used to treat patients has been based on in vitro studies of drug efficacy against clinical isolates of B. mandrillaris or on empirical treatment of surviving cases. The core antimicrobials include pentamidine, miltefosine, albendazole, and fluconazole/itraconazole, and survivors have usually received a combination of macrolide antibiotics (azithromycin or clarithromycin), azole antibiotics (fluconazole or itraconazole), pentamidine, sulfadiazine, and miltefosine.4,18,29 Amphotericin B is an effective agent against Naegleria species, but is inferior against B. mandrillaris isolates.30,31 Failure of antimicrobial therapy is attributed mainly to poor CSF penetration and the thick cell wall of the amebic cyst. Therefore, new drugs that minimize toxicity across the BBB and with amoebicidal activity are needed urgently. A recent study identified novel potent nanomolar inhibitors and micromolar inhibitors, including Milciclib maleate, Thimerosol, Dabrafenib mesylate, and LTX-315, etc. These compounds show promise as new targets for validating structure-based drug design.32 Inspiring some promising studies have conjugated existing novel azole compounds, or the natural phenol curcumin with nanoparticles further enhanced the ability of the compounds against B. mandrillaris, leading to better treatments for GAE cases; however, future in vivo studies are required.33 The prolonged approval process for the clinical application of compounds and the rarity of the disease has hindered the development of effective therapies. Drugs with a known mode of action for clinical use are considered a viable approach, and given that glucose is an important component of B. mandrillaris cyst wall composition and/or its biosynthesis, studies have evaluated a range of hypoglycemic agents such as indaziflam, nateglinide, acarbose as well as glimepiride and have demonstrated their amoebicidal properties.34 However, it is not yet known whether these drugs will be approved clinically.
Interestingly, the progressive exacerbation of neurological symptoms was transiently controlled after linezolid treatment. In vitro studies have shown that linezolid binds to sites on 23S ribosomal RNA (rRNA) of the bacterial 50S subunit, thereby preventing the formation of a functional 70S initiation complex, which is essential for bacterial as well as B. mandrillaris multiplication. Although the in vitro antimicrobial spectrum of linezolid did not cover protozoa, the authors concluded that its antimicrobial spectrum needs further mechanistic exploration by in vitro studies.
Conclusion
Early diagnosis of B. mandrillaris infection is difficult, and a history of trauma and dirty water or soil exposure should be of concern in patients with suspected BAE. In addition, more transmission routes such as organ transplants and application of water tanks should be concerned. Specific skin features facilitate early diagnosis and increase the chances of survival. The limitation of our study is that we failed to perfect the spinal lesion and skin biopsy, and gastrointestinal examination. To identify the causative pathogenic microorganism, we suggest the unbiased metagenomic sequencing of the affected tissues/CSF in patients with CNS infections that are difficult to diagnose or treat, including amoebic encephalitis, multiple tests at different stages of the disease may be required in these patients. Despite the suboptimal efficacy of conventionally available drugs in the treatment, some emerging and promising studies coupling existing drugs to nanoparticles, novel therapies utilizing novel azole classes, or hypoglycemic drugs possessing anti-amoebic activities have been reported. We appeal to the urgent need for collaboration between academia, the pharmaceutical industry, and water companies to prevent and treat this deadly infection.
Ethics and Consent
This report has been approved by the affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine’s ethics committee consent to publish the case details. Written informed consent to have the case details published has been provided by the patient’s son.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Visvesvara GS, Martinez AJ, Schuster FL, et al. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol. 1990;28:2750–2756. doi: 10.1128/jcm.28.12.2750-2756.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x [DOI] [PubMed] [Google Scholar]
- 3.Bhosale NK, Parija SC. Balamuthia mandrillaris: an opportunistic, free-living ameba - An updated review. Trop Parasitol. 2021;11(2):78–88. doi: 10.4103/tp.tp_36_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin Infect Dis. 2003;37(10):1304–1312. doi: 10.1086/379020 [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui R, Khan A. Current treatment options of Balamuthia mandrillaris: a patent overview. Pharm Pat Anal. 2020;9(4):121–123. doi: 10.4155/ppa-2020-0016 [DOI] [PubMed] [Google Scholar]
- 6.Kum SJ, Lee HW, Jung HR, et al. Amoebic encephalitis caused by Balamuthia mandrillaris. J Pathol Transl Med. 2019;53(5):327–331. doi: 10.4132/jptm.2019.05.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baquero RA, Reyes-Batlle M, Nicola GG, et al. Presence of potentially pathogenic free-living amoebae strains from well water samples in Guinea-Bissau. Pathog Glob Health. 2014;108(4):206–211. doi: 10.1179/2047773214Y.0000000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunnebacke TH, Schuster FL, Yagi S, et al. Balamuthia mandrillaris from soil samples. Microbiology. 2004;150(Pt 9):2837–2842. doi: 10.1099/mic.0.27218-0 [DOI] [PubMed] [Google Scholar]
- 9.Niyyati M, Karamati SA, Lorenzo MJ, et al. Isolation of Balamuthia mandrillaris from soil samples in North-Western Iran. Parasitol Res. 2016;115(2):541–545. doi: 10.1007/s00436-015-4770-y [DOI] [PubMed] [Google Scholar]
- 10.Bravo F, Sanchez MR. New and re-emerging cutaneous infectious diseases in Latin America and other geographic areas. Dermatol Clinics. 2003;21(4):655–668. doi: 10.1016/S0733-8635(03)00090-1 [DOI] [PubMed] [Google Scholar]
- 11.Farnon EC, Kokko KE, Budge PJ, et al. Transmission of Balamuthia mandrillaris by Organ Transplantation. Clin Infect Dis. 2016;63(7):878–888. doi: 10.1093/cid/ciw422 [DOI] [PubMed] [Google Scholar]
- 12.Kiderlen AF, Laube U. Balamuthia mandrillaris, an opportunistic agent of granulomatous amebic encephalitis, infects the brain via the olfactory nerve pathway. Parasitol Res. 2004;94(1):49–52. doi: 10.1007/s00436-004-1163-z [DOI] [PubMed] [Google Scholar]
- 13.Kiderlen AF, Laube U, Radam E, Tata PS. Oral infection of immunocompetent and immunodeficient mice with Balamuthia mandrillaris amebae. Parasitol Res. 2007;100(4):775–782. doi: 10.1007/s00436-006-0334-5 [DOI] [PubMed] [Google Scholar]
- 14.Cope JR, Landa J, Nethercut H, et al. The epidemiology and clinical features of Balamuthia mandrillaris disease in the United States, 1974–2016. J Clin Microbiol. 2019;68(11):1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez AJ, Visvesvara GS. Balamuthia mandrillaris infection. J Med Microbiol. 2001;50(3):205–207. doi: 10.1099/0022-1317-50-3-205 [DOI] [PubMed] [Google Scholar]
- 16.Matin A, Stins M, Kim KS, Khan NA. Balamuthia mandrillaris exhibits metalloprotease activities. FEMS Immunol Med Microbiol. 2006;47(1):83–91. doi: 10.1111/j.1574-695X.2006.00065.x [DOI] [PubMed] [Google Scholar]
- 17.Matin A, Nawaz S, Jung SY. Report: effect of macrophage alone or primed with cytokines on Balamuthia mandrillaris interactions with human brain microvascular endothelial cells in vitro. Pak J Pharm Sci. 2018;31(6):2553–2559. [PubMed] [Google Scholar]
- 18.Lorenzo-Morales J, Cabello-Vílchez AM, Martín-Navarro CM, Martínez-Carretero E, Piñero JE, Valladares B. Is Balamuthia mandrillaris a public health concern worldwide? Trends Parasitol. 2013;29(10):483–488. doi: 10.1016/j.pt.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Cheng W, Li B, et al. Balamuthia mandrillaris infection in China: a retrospective report of 28 cases. Emerg Microbes Infect. 2020;9(1):2348–2357. doi: 10.1080/22221751.2020.1835447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, Zhang Y, Yu Y, et al. Encephalomyelitis caused by Balamuthia mandrillaris in a woman with breast cancer: a case report and review of the literature. Front Immunol. 2022;12:768065. doi: 10.3389/fimmu.2021.768065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabello-Vílchez AM, Rodríguez-Zaragoza S, Piñero J, Valladares B, Lorenzo-Morales J. Balamuthia mandrillaris in South America: an emerging potential hidden pathogen in Perú. Exp Parasitol. 2014;145:S10–S19. doi: 10.1016/j.exppara.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 22.Bravo FG, Cabrera J, Gottuzo E, Visvesvara GS. Cutaneous manifestations of infection by free-living amebas. Trop Dermatol. 2006;50:49–55. [Google Scholar]
- 23.Yang Y, Hu X, Min L, et al. Balamuthia mandrillaris-related primary amoebic encephalitis in China diagnosed by next generation sequencing and a review of the literature. Lab Med. 2020;51(2):e20–e26. doi: 10.1093/labmed/lmz079 [DOI] [PubMed] [Google Scholar]
- 24.Healy JF. Balamuthia amebic encephalitis: radiographic and pathologic findings. AJNR Am J Neuroradiol. 2002;23(3):486–489. [PMC free article] [PubMed] [Google Scholar]
- 25.Duke BJ, Tyson RW, DeBiasi R, Freeman JE, Winston KR. Balamuthia mandrillaris meningoencephalitis presenting with acute hydrocephalus. Pediatr Neurosurg. 1997;26(2):107–111. doi: 10.1159/000121172 [DOI] [PubMed] [Google Scholar]
- 26.Ong TYY, Khan NA, Siddiqui R, Kraft CS. Brain-eating amoebae: predilection sites in the brain and disease outcome. J Clin Microbiol. 2017;55(7):1989–1997. doi: 10.1128/JCM.02300-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Detering H, Aebischer T, Dabrowski PW, et al. First draft genome sequence of Balamuthia mandrillaris, the causative agent of amoebic encephalitis. Genome Announc. 2015;3(5):e01013–e01015. doi: 10.1128/genomeA.01013-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armangue T, Spatola M, Vlagea A, et al. Spanish Herpes Simplex Encephalitis Study G Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760–772. doi: 10.1016/S1474-4422(18)30244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shehab KW, Aboul-Nasr K, Elliott SP. Balamuthia mandrillaris granulomatous amebic encephalitis with renal dissemination in a previously healthy child: case report and review of the pediatric literature. J Pediatr Infect Dis Soc. 2018;7(3):e163–e168. doi: 10.1093/jpids/pix089 [DOI] [PubMed] [Google Scholar]
- 30.Seidel JS, Harmatz P, Visvesvara GS, Cohen A, Edwards J, Turner J. Successful treatment of primary amebic meningoencephalitis. N Engl J Med. 1982;306(6):346–348. doi: 10.1056/NEJM198202113060607 [DOI] [PubMed] [Google Scholar]
- 31.Schuster FL, Visvesvara GS. Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amebic meningoencephalitis in humans and other animals. J Clin Microbiol. 1996;34(2):385–388. doi: 10.1128/jcm.34.2.385-388.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice CA, Colon BL, Chen E, Hull MV, Kyle DE. Discovery of repurposing drug candidates for the treatment of diseases caused by pathogenic free-living amoebae. PLoS Negl Trop Dis. 2020;14(9):e0008353. doi: 10.1371/journal.pntd.0008353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anwar A, Mungroo MR, Khan S, et al. Novel azoles as antiparasitic remedies against brain-eating amoebae. Antibiotics. 2020;9(4):188. doi: 10.3390/antibiotics9040188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui R, Mungroo MR, Anuar TS, et al. Antiamoebic properties of laboratory and clinically used drugs against Naegleria fowleri and balamuthia mandrillaris. Antibiotics. 2022;11(6):749. doi: 10.3390/antibiotics11060749 [DOI] [PMC free article] [PubMed] [Google Scholar]