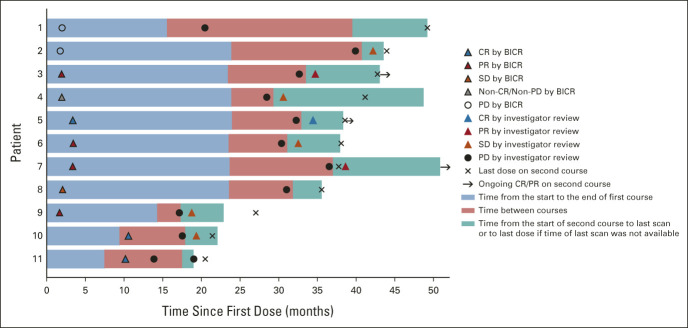
FIG 5.
Pembrolizumab second-course response characteristics.a-c Each bar represents one patient who received a second course of pembrolizumab. Shown here are first-course first objective response per RECIST v1.1 by BICR, progressive disease after stopping first course per RECIST v1.1 by investigator review, and second-course objective response per RECIST v1.1 by investigator review. Eligibility for second course and response during second course were assessed by the investigator (not by BICR). aAt data cutoff, patients 1 and 8 did not have available last scan on second-course pembrolizumab. bPatients 2, 4, 5, 6, 7, and 8 received first-course treatment of pembrolizumab alone. Patients 1, 3, 9, 10, and 11 received first-course pembrolizumab-chemotherapy. cPatient 1 discontinued first-course pembrolizumab-chemotherapy with CR before PD occurred. At the time of PD, the patient's lesions were smaller than 1 cm and the patient did not have any symptoms of progression. Therefore, the investigator's plan, as agreed on by the study sponsor, was to repeat scans per the protocol schedule and start second-course pembrolizumab once lesions were larger than 1 cm. BICR, blinded independent central review; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.