Figure 2.
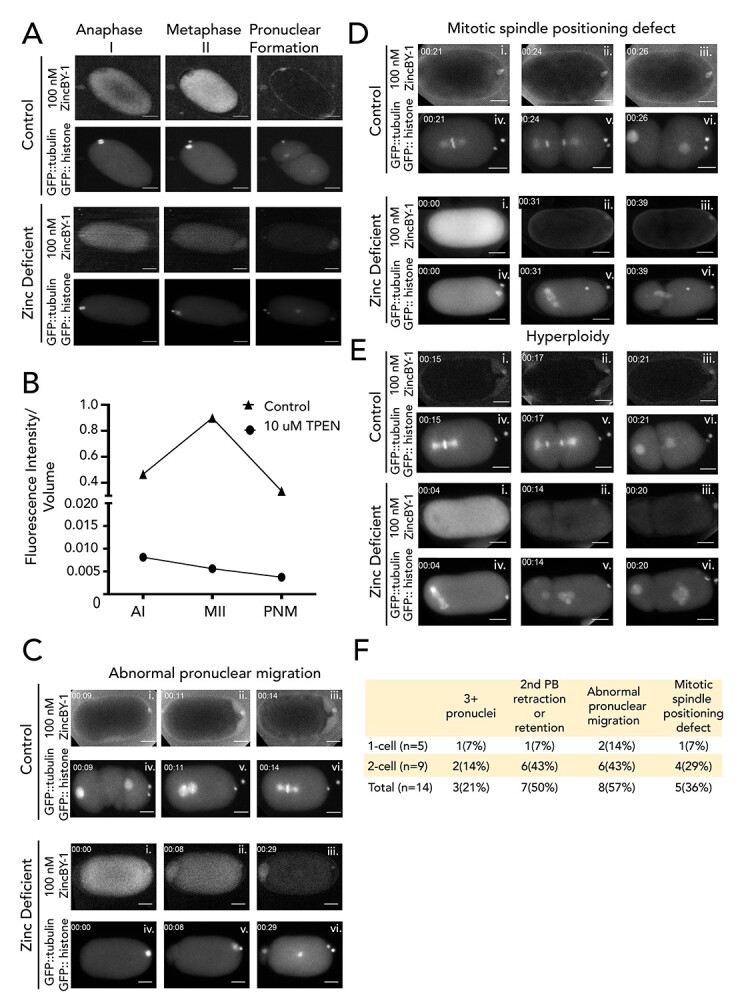
Blocking zinc influx with TPEN induces a series of meiotic defects. Fluorescence images are of embryos expressing GFP::histone and GFP::tubulin (bottom row of images) and treated with 100 nM ZincBY-1 (top row of images). (A, B) Control embryos that cycle through the meiotic divisions show an increased uptake of ZincBY-1 fluorescence from Anaphase I to Metaphase II. Compared with controls, embryos treated with 10 μM TPEN at Anaphase I prior to eggshell formation show decreased ZincBY-1 fluorescence intensity that continuously drops through pronuclear formation. (C–E) Embryos were dissected into 10 μM TPEN, mounted, and filmed. T = 0 represents the beginning of filming. ZincBY-1 and GFP pairs are from the same movie. (C) A pronucleus is trapped in the posterior end of the embryo and is contained within a small portion of the cytoplasm as ZincBY-1 fluorescence decreases (i–iii) and the cell cycle progresses (iv–vi). (D) iv–vi shows a mitotic spindle in the posterior end of the embryo oriented perpendicularly, demonstrating defects in spindle positioning. ZincBY-1 fluorescence also decreases (i–iii). (E) iv–vi shows a misoriented mitotic spindle and extra nuclei as a result of mitotic defects, whereas ZincBY-1 fluorescence decreases during pronuclear migration (i–iii). (F) All embryos were collected in five experiments (n = 14) and were exposed to 10 μM TPEN contained meiotic defects: 21% (n = 3/14) contained 3 or more pronuclei, 50% (n = 7/14) had retraction or retention of the second polar body, 36% (n = 5/14) had mitotic spindle positioning defects, and 57% (n = 8/14) had abnormal pronuclear migration leading to hyperploidy. Despite the defects, the embryos could still progress to the 1-cell (n = 5) or 2-cell stage (n = 9). All scale bars = 10 μm.
