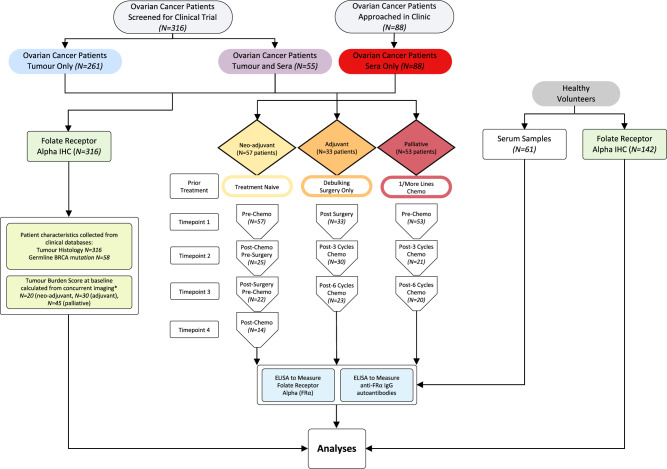
Fig. 1. Study design and sample workflow.
Serum samples from ovarian cancer patients were collected at timepoint 1 and up to 3 sequential treatment-related time points (e.g., for the treatment-naïve group: pre-chemo, post-chemo/pre-surgery, post-surgery/pre-chemo, and post-chemo). These were collected alongside serum samples from healthy volunteers. Serum samples were studied for sFRα and anti-FRα autoantibodies, and FRα protein expression in patient tumours and normal tissue microarrays was evaluated by immunohistochemistry. Patient characteristics, including tumour histotype, were collected from clinical databases (see Supplementary Table 1 for ovarian patients in the longitudinal study). Patient tumour burden scores were calculated from concurrent imaging (*see Supplementary Table 2 for a scoring system). N numbers are indicated in the figure.