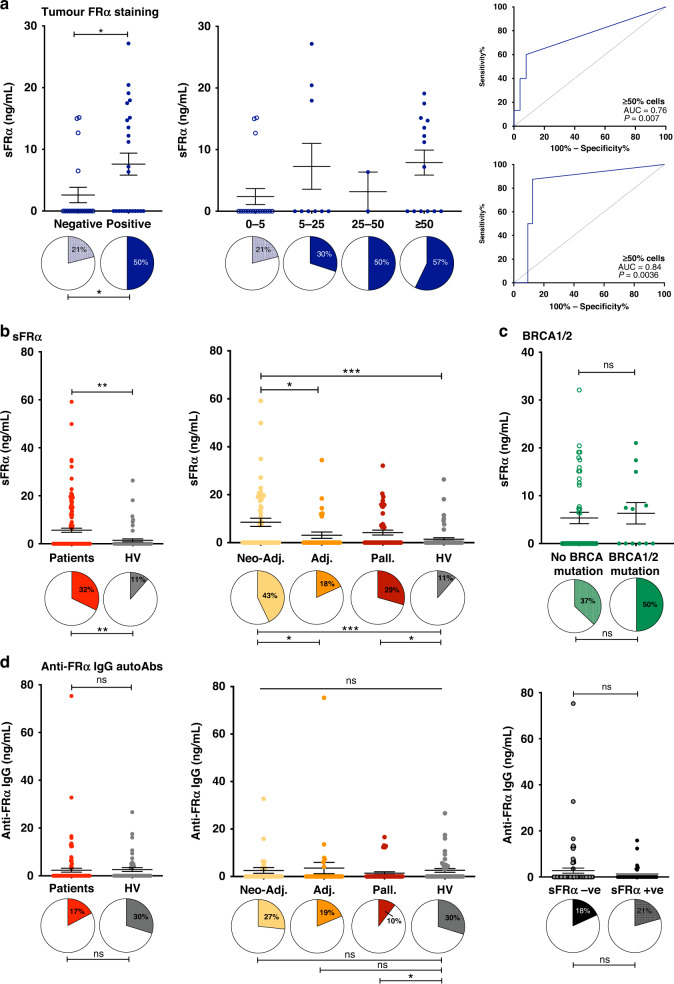
Fig. 4. Soluble FRα but not anti-FRα autoantibodies were elevated in the circulation of ovarian cancer patients compared with healthy subjects.
sFRα and anti-FRα autoantibodies were measured in three patient cohorts (neo-adjuvant, adjuvant, palliative) at timepoint 1 (see Fig. 1). a sFRα levels were significantly-higher in patients with FRα tumour cell membrane expression, compared to patients with FRα-negative tumours (left) and with trend for higher levels in patients with tumours showing FRα cell membrane expression in a greater proportion of tumour cells (middle). In neo-adjuvant treatment-naïve patients, baseline sFRα concentration was predictive of FRα cell membrane expression in both ≥5% and ≥50% of tumour cells (right top and bottom, respectively). b Significantly-higher sFRα levels were measured in ovarian cancer patients compared to healthy volunteers, and between patient cohorts (proportion of samples with detectable sFRα indicated by filled pie chart sections below). c sFRα levels, or proportions of samples with detectable sFRα, were not associated with the patient’s germline BRCA1/2 mutational status. d Comparable levels of anti-FRα autoantibodies were detected in serum samples from ovarian cancer patients and healthy volunteers (left), across all patient cohorts (middle), and irrespective of detectable sFRα (right). N numbers in Fig. 1; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Statistical tests: t test, one-way ANOVA with Kruskal–Wallis multiple comparisons, receiver operating characteristic (ROC) curve analyses and Chi square test. Error bars represent the standard error of mean (SEM).