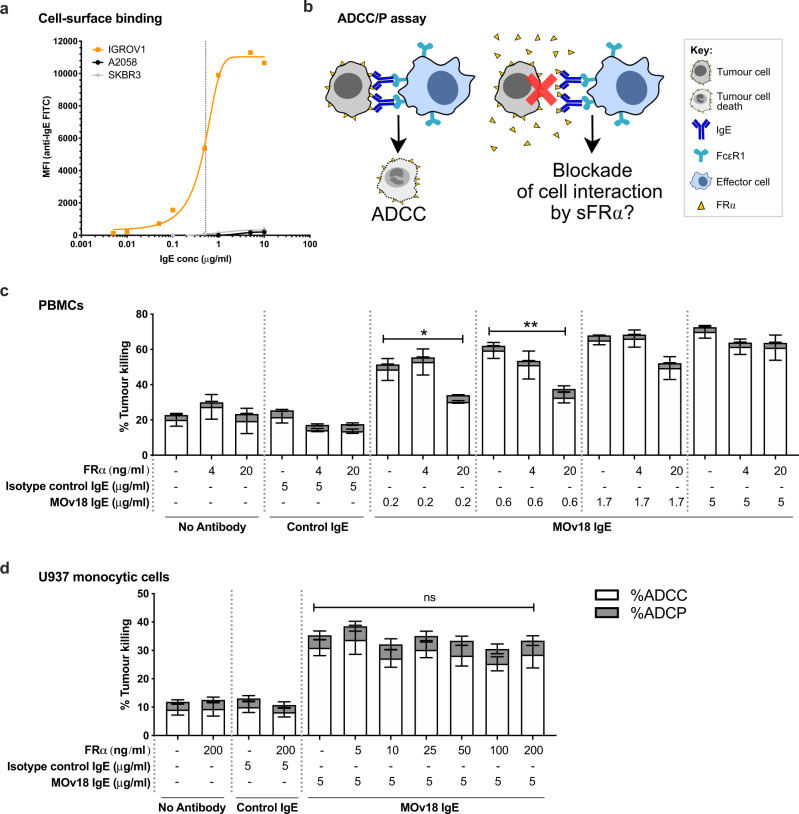
Fig. 6. Potential blockade of efficacy of a FRα-targeted therapeutic antibody candidate by soluble FRα.
a Binding of MOv18 IgE to FRα-expressing IGROV1 ovarian, but not to melanoma (A2058) or breast (SKBR3), cancer cells (EC50 = 0.53 µg/ml for binding to IGROV1 cells, indicated by vertical dotted line). b Schematic of potential blockade of MOv18 IgE anti-tumour function by sFRα. c Using PBMCs, the level of antibody-dependent cellular cytotoxicity (ADCC) mediated by the FRα-specific antibody MOv18 IgE was significantly reduced by a high concentration of FRα antigen (20 ng/ml) where MOv18 IgE was introduced at non-saturating concentrations of cancer cell-associated FRα (0.2 and 0.6 µg/ml; below or at the EC50 as shown in a). High concentrations of FRα antigen (20 ng/ml) did not block ADCC mediated by concentrations of MOv18 IgE above the EC50 (see a). d Similarly, with U937 monocytic cells, ADCC mediated by a saturating concentration of MOv18 IgE (5 µg/ml; see a) was not blocked by supraphysiological concentrations of FRα antigen (200 ng/ml). Antibody-dependent cellular phagocytosis (ADCP) was not mediated by MOv18 IgE. N = 6 and 4 independent experiments, respectively. *P ≤ 0.05, **P ≤ 0.01, ns not significantly different. Statistical test: one-way ANOVA with Kruskal–Wallis multiple comparisons. Error bars represent the standard error of mean (SEM).