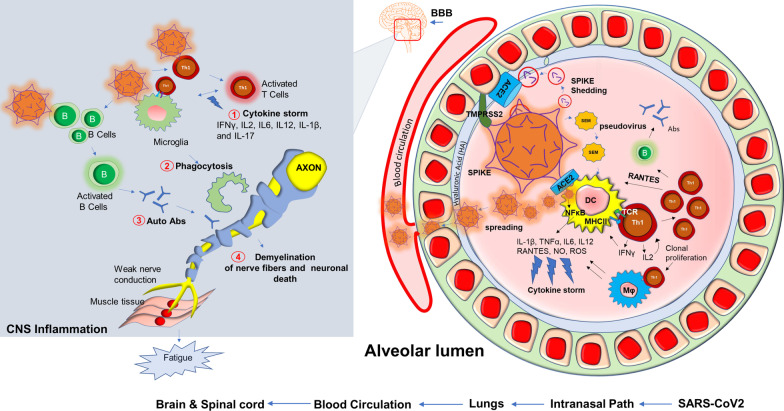
Fig. 5.
Potential inflammatory pathways in muscle fatigue of long-haul COVID patients. Upon entry of SARS-CoV2, a possible cascade of acute inflammatory pathways in the alveolar lumen was displayed. SARS-CoV2 employs its Spike protein or S-glycoprotein to bind with ACE2 receptor and membrane-bound serine protease TMPRSS2. SARS-CoV2 also interacts with hyaluronan or hyaluronic acid of the glycocalyx layer. SEM (Spike, Envelope, Membrane) pseudovirus particles or potential possible shedding of spike proteins also cause direct infection in alveolar dendritic cells followed by MHC-II presentation and activation of CD4 + Th1 cells. Subsequent production of IFNγ and virus-induced activation of NF-κB might evoke productions of inflammatory cytokines and chemokines commonly known as cytokine storm (#1). Th1 cell-mediated severe activation of Mφ and microglia might also cause non-specific phagocytosis of myelin (#2). Possible activation of B cells produces autoantibodies (#3). Eventually, active virus particles, T cells, and inflammatory mediators spread through distant organs across BBB, and cause a cell-based inflammatory response resulting demyelinating effects in the central and peripheral nervous system (#4). Impaired nerve signal causes muscular fatigue. B = B cells; T = T cells; Abs = antibodies; APCs = antigen-presenting cells