Abstract
We describe four new nodulose-spored species of Inocybe from tropical regions of Africa: I. beninensis, I. flavipes, I. fuscobrunnea and I. pallidiangulata. The new species are recognised based on morphological data and phylogenetic analyses of ITS, 28S and RPB2 sequences. Phylogenetic analyses indicated that I. flavipes and I. beninensis are part of a subclade leading to the I. calida group. Inocybe fuscobrunnea appears sister to the I. asterospora group. Inocybe pallidiangulata is nested within a clade of mainly tropical species from South Asia, Africa, and South America, close to the subclade of I. lilacinosquamosa and I. ayangannae from Guyana. Complete descriptions and illustrations, including photographs and line drawings, and a key to nodulose-spored taxa of tropical African species of Inocybe are provided.
Citation: Aïgnon HL, Jabeen S, Verbeken A, Matheny PB, Yorou NS, Ryberg M (2022). Four new nodulose-spored species of Inocybe (Agaricales) from West Africa. Fungal Systematics and Evolution 10: 1–18. doi: 10.3114/fuse.2022.10.01
Keywords: Agaricomycetes, ectomycorrhizal fungi, Inocybaceae, molecular phylogeny, new taxa, systematics, taxonomy
INTRODUCTION
Inocybe is the most diverse genus in the Inocybaceae with 850 species described worldwide (Matheny et al. 2020), but this number has increased as more areas are intensively studied and new species described (Matheny & Bougher 2017, Matheny & Kudzma 2019, Bandini & Oertel 2020, Caiafa et al. 2021). While competing infrageneric classification systems are available based on morphological analyses (Kuyper et al. 1986, Singer 1986, Bon 1997, 1998), these classifications are a poor representation of the phylogenetic relations within the group. For example, nodulose-spored species of the genus do not form a monophyletic group (Matheny et al. 2002, Matheny 2005, Kropp et al. 2010, Ryberg et al. 2010, Esteve-Raventós et al. 2016).
Anatomically, species of Inocybe are distinguished from those belonging to other genera of the family by possession of pleurocystidia and/or angular, nodulose, or spinose basidiospores, often with a distinct apiculus (Matheny et al. 2020). Some subgroups of Inocybe lack caulocystidia, whereas others possess them near the apex or along the entire length of the stipe correlated with the presence/absence of a partial veil (Kuyper 1986).
Despite progress to assess the diversity of Inocybe in many parts of the world, African regions remain poorly explored (Aïgnon et al. 2021a). To date, only four nodulose-spored species of Inocybe have been recorded from Africa. Hennings (1902) described I. cyaneovirescens, from what used to be called “German East Africa”, which today encompasses the nations of Burundi, Rwanda and continental region of Tanzania. Pegler (1969) described I. ghanaensis from West Africa (Ghana). Lastly, Buyck & Eyssartier (1999) described two species I. conspicuospora and I. glaucodisca from Zambia. In addition to these, two studies published sequence data from several undescribed species from Zambia (Matheny et al. 2009, Tedersoo et al. 2011). In recent years, eight Inocybe species were recorded from Morocco (Ouabbou et al. 2014, Akil et al. 2015). Recently, Aïgnon et al. (2021b) estimated that approximately 62 species of Inocybe occur in Africa.
According to Matheny et al. (2020), certain regions such as Mediterranean types of habitats in North America and Australia are predominantly rich in species of Inocybe with smooth spores (Nishida 1989, Matheny & Bougher 2017), but tropical regions have accumulated a diverse assemblage of species with angular-nodulose spores (Matheny et al. 2003, Horak et al. 2015). This seems indeed to be the case in tropical Africa where we discovered four new species of Inocybe, all with angular-nodulose spores. Detailed descriptions and illustrations, as well as comparisons with closely related species, are provided. A key to nodulose-spored species of Inocybe from tropical Africa is also provided.
MATERIAL AND METHODS
Study area and specimen sampling
Specimens were collected between 2013 and 2018 in Benin, Burkina Faso, Ivory Coast and Togo. Collections were made in woodlands, with more than 10 % woody cover, including shrublands with a canopy only 2 m high, and that were dominated by ectomycorrhizal trees of Euphorbiaceae, Fabaceae and Phyllanthaceae. Colour codes of fresh specimens were recorded with the Online Auction Colour chart (2004). After labelling and recording morphological data, specimens were preserved by drying using an electric dryer (type Stöckli Dörrex) for 24 h at 45 °C. All studied materials, including the holotypes were deposited at the Mycological Herbarium of Parakou University, Benin Republic (UNIPAR).
Morphological and anatomical analyses
Fine sections from the dried basidiomata were rehydrated and examined in 3 % KOH and Congo Red for microscopic investigation. Drawings of microscopic characters were made with the aid of a drawing tube attached to a Leica DM2700 light microscope. Microscopic characters were drawn at 1000× magnification. For each species, spore measurements were made from 40 spores. We measured length (L) and width (W) of the basidiospores and calculated the ratio Q = L/W. Measurements of basidiospores and basidia excluded the apiculus and sterigmata, and spore dimensions included nodules. Spore measurements are given as (a–)b–c(–d), where (a) = extreme minimum value, range b–c contains minimum of 90 % of the calculated values and (d) = extreme maximum value following Aïgnon et al. (2021a, c).
Molecular analyses
DNA extraction, PCR and sequencing: Genomic DNA was extracted from dried specimens by a QIAGEN® plant mini kit. The ITS, parts of 28S, and RPB2 were amplified. For the ITS region, we produced amplicons using primers pairs ITS1F and ITS4 (White et al. 1990, Gardes & Bruns 1993). For the 28S region, we used the LR0R, LR7, LR5 and LR3R primers (Vilgalys & Hester 1990, Cubeta et al. 1991, Rehner & Samuels 1995) and for the RPB2 region, primer pairs b6F and b7.1R (Matheny 2005) were used. PCR products were purified and sequenced at Macrogen Inc. (Netherlands) using the same primers as those used for PCR. We refer to Aignon et al. (2021a, c) for detailed methods of the DNA extraction and amplification.
Sequence alignment and phylogenetic analyses: New sequences derived in this study were compared with closely related Inocybe sequences that were retrieved from GenBank (Benson et al. 2010). Species of different groups, clades and sections of Inocybe such as I. praetervisa group, I. mixtilis group, I. napipes group, I. xanthomelas group, I. diabolica group, I. lacera group, I. lanuginosa group, I. giacomi group, Smooth-spored temperate boreal clade, Smooth-spored temperate boreal clade and Sect. Inocybe were selected on the basis of a literature survey (Ryberg et al. 2008, Horak et al. 2015, Matheny & Bougher 2017, Matheny et al. 2017, Latha & Manimohan 2017, Esteve-Raventós et al. 2018, Bandini et al. 2019, Cripps et al. 2019, Matheny et al. 2020). Sequences of the different regions (ITS, 28S and RPB2) were aligned separately using MAFFT v. 7.464 (Katoh et al. 2019), and a final concatenated data set of ITS, 28S and RPB2 was generated using Geneious v. 7.0.2 software (Biometer, Auckland, New Zealand). The dataset was partitioned into ITS + 28S, RPB2 codon position 1 + RPB2 codon position 2 and RPB2 codon position 3 + the intron in RPB2 separately. For phylogenetic analyses, the substitution models and the best partitioning schemes were determined for both Maximum Likelihood (ML) and Bayesian Inference (BI). The substitution models for each locus were determined based on the AICc model selection criterion as implemented in PartitionFinder (Lanfear et al. 2016).
Maximum Likelihood analyses were performed with IQ-TREE v. 1.6.12 (Nguyen et al. 2015). Ultrafast bootstrapping (UFBoot) was done with 1 000 replicates (Hoang et al. 2017). BI analyses were performed in MrBayes v. 3.2.7a (Ronquist et al. 2012) using a GTR+I+G model at the Cipres Science Gateway (Miller et al. 2010). Two independent Markov Chain Monte Carlo (MCMC) were run in parallel, each with four chains for 20 M generations. Posterior probabilities (BPP) were calculated after discard the first 25 % samples from the cold chain by default. Sequences of Nothocybe distincta were used for rooting purposes based on Matheny et al. (2020). Nodes that received bootstrap proportions, ≥ 80 % of SHaLRT support, ≥ 95 % of UFBoot support and > 0.95 of BPP support were considered strongly supported, and nodes that met the criteria for strong support for at least one, but not all three of the methods, were considered moderately supported. Phylogenetic reconstructions for gene regions (ITS+28S, RPB2) was also performed separately with IQ-TREE v. 1.6.12.
RESULTS
Phylogenetic analysis
This study generated 17 new sequences submitted to GenBank (Table 1). The new species named Inocybe beninensis failed to yield any RPB2 amplicons. In the dataset, the ITS partition included 150 taxa and 925 sites, the 28S partition included 163 taxa and 1 529 sites and RPB2 included 85 taxa and 782 sites. Individual gene tree phylogenies (ITS+28S and RPB2) are shown in Supplementary Figs S1, S2. No strongly supported conflict was observed. Phylogenetic analyses of ITS, 28S and RPB2 sequences data supported the distinction of four novel nodulose-spored Inocybe from tropical regions of Africa described below as I. beninensis sp. nov., I. flavipes sp. nov., I. fuscobrunnea sp. nov. and I. pallidiangulata sp. nov. All four new species were well-separated from sister species, and separate collections of the same species formed well supported clades with short internal branches in ML and BI analyses (Fig. 1).
Table 1.
List of taxa used in the molecular analyses along with vouchers, GenBank accession numbers and geographic origin. The new species and their accession numbers are in bold.
| Species | Voucher | Country |
GenBank accession no.
|
References | ||
|---|---|---|---|---|---|---|
| ITS | 28S | RPB2 | ||||
| Inocybe acanthosperma Matheny & Bougher | PBM3773 | Australia | KJ729861 | KJ729889 | KJ729923 | Matheny et al. (2017) |
| I. acuta Boud | TURA:5066 | Finland | KP171102 | KM197208 | n/a | Unpublished |
| I. aff. asterospora Quél | PBM2453, PBM-2014 | USA | DQ404390 | AY702015 | n/a | Kuo & Matheny (2015) |
| I. aff. diabolica Vauras | PBM2976 | USA | n/a | KP170948 | KM246001 | Horak et al. (2015) |
| I. aff. xanthomelas Boursier & Kühner | PAM06060405, TENN063834 | France | HQ586867 | HQ641109 | n/a | Horak et al. (2015) |
| I. alpinomarginata C.L. Cripps, E. Larss.& Vauras | CLC1303 | USA | MK153644 | MK153644 | n/a | Cripps et al. (2019) |
| I. ambigua Romagn | BJ910730 | Sweden | AM882800 | AM882800 | n/a | Ryberg et al. (2008) |
| I. angustifolia (Corner & E. Horak) Garrido | DED8139 | Thailand | GQ892988 | GQ892942 | MH577422 | Horak et al. (2015) |
| I. antoniniana E. Sesli, Bandini & Krisai | Fungi 4064 | Turkey | MN988712 | n/a | n/a | Bandini et al. (2020b) |
| I. appendiculata Kühner | SAT0026155 | USA | n/a | JN974946 | MH577432 | Ryberg & Matheny (2012) |
| I. arctica E. Larss., Vauras & C.L. Cripps | JV2238 | Norway | KY033843 | KY033843 | n/a | Larsson et al. (2017) |
| I. argenteolutea Vauras | EL9906 | Sweden | FN550889 | FN550889 | n/a | Ryberg et al. (2010) |
| I. asterospora Quél | EL100-14 | Sweden | MN296110 | MN296110 | n/a | Cripps et al. (2019) |
| I. ayangannae Matheny, Aime & T.W. Henkel | MCA 1232 | Guyana | n/a | AY239018 | AY337364 | Matheny et al. (2009) |
| I. babruka K.P.D. Latha & Manim. | CAL 1344 | India | KY440086 | KY549116 | KY553237 | Latha & Manimohan (2017) |
| I. baltica Vauras & E. Larss. | EL50-09 | Svalbard | KY033838 | KY033838 | n/a | Larsson et al. (2017) |
| I. beninensis Aïgnon, Yorou & Ryberg | HLA0390 | Benin | MN096196 | MN097888 | n/a | This study |
| HLA0467 | Benin | MT994602 | n/a | n/a | ||
| I. bombina Bandini & B. Oertel | KR-M-0043212 | Germany | MK929261 | n/a | n/a | Bandini et al. (2019) |
| I. botaurina Bandini & B. Oertel | DB1-6-12-1 | Germany | MK929259 | n/a | n/a | Bandini et al. (2019) |
| I. brevisquamulosa E. Horak, Matheny & Desjardin | ZT10102 | Thailand | NR_153123 | GQ892974 | n/a | Horak et al. (2015) |
| I. brunneolipes Grund & D.E. Stuntz | DG1863 | Canada | KY923032 | NG_057289 | n/a | Unpublished |
| I. cacaocolor Matheny & Bougher | PBM3790 | Australia | KJ778845 | KJ756464 | KJ756422 | Matheny et al. (2017) |
| I. calida Valen | TAA185175 | Estonia | AM882760 | AM882760 | n/a | Ryberg et al. (2008) |
| I. calocephala Matheny & Bougher | PBM3600 | Australia | n/a | NG 057234 | KJ756413 | Matheny & Bougher (2017) |
| I. calospora f. pectinata Guinb | PAM10082903 | France | KP171112 | KP170900 | n/a | Unpublished |
| I. calospora Quél | PAM08092808 | France | n/a | KP170899 | n/a | Unpublished |
| I. calospora Quél | PAM99082905 | France | HQ586871 | HQ641114 | n/a | Unpublished |
| I. calospora Quél | PAM10092501 | France | KP171113 | KP170901 | n/a | Matheny et al. (2017) |
| I. calospora Quél | PAM00073102 | France | HQ586853 | HQ641093 | n/a | Unpublished |
| I. calospora Quél | PAM03082401 | France | HQ586852 | Q641094 | n/a | Unpublished |
| I. calospora Quél | EL9505 | Finland | AM882759 | AM882759 | n/a | Ryberg et al. (2008) |
| I. candidipes Kropp & Matheny | BK 24-July-99-7 | USA | n/a | AY239019 | AY337366 | Kropp & Matheny (2004) |
| I. caprimulgi Vauras & E. Larss. | JV5808 | Finland | KT958924 | KT958924 | n/a | Vauras & Larsson (2016) |
| I. cerasphora Singer | BSI 01/184 | Chile | n/a | AY380370 | AY337367 | Matheny (2005) |
| I. ceskae Bandini | PBM 1315 | USA | n/a | AY380387 | AY337395 | Matheny (2005) |
| I. cf. intricata Peck | TENN:063834 | France | n/a | KP170914 | KM245990 | Matheny & Bougher (2017) |
| I. cf. xanthomelas Boursier & Kühner | EL3505 | Norway | AM882989 | AM882989 | n/a | Ryberg et al. (2008) |
| I. chalcoceps Matheny & Bougher | TENN:068946 | Australia | n/a | NG 057228 | n/a | Matheny & Bougher (2017) |
| I. chondroderma D.E. Stuntz ex Matheny, Norvell & E.C. Giles | PBM1776 | USA | GU949579 | JN974967 | MH249789 | Matheny et al. (2013) |
| I. conspicuospora Buyck & Eyssart. | PC 96042 | Zambia | n/a | EU555471 | EU555470 | Matheny et al. (2009) |
| I. corydalina Quél | TURA6488/AM10687 | Belgium/Russia | AY038314 | AY337370 | n/a | Matheny et al. (2002) |
| I. curvipes P. Karst | PBM 2401 | USA | n/a | AY239022 | AY337414 | Matheny (2005) |
| I. diabolica Vauras | EL9006 | Sweden | FN550896 | FN550896 | n/a | Ryberg et al. (2010) |
| I. dunensis P.D. Orton | EL22906 | France | FN550888 | FN550888 | n/a | Unpublished |
| I. epidendron Matheny, Aime & T.W. Henkel | MCA 1880, TH9186 | Guyana | JN168725 | EU569840 | n/a | Matheny et al. (2009) |
| I. eriocaulis Matheny & Bougher | PBM2132 | Australia | KJ778853 | EU569843 | EU569842 | Matheny et al. (2009) |
| I. favrei Bon | JV30673 | Norway | KY033798 | KY033798 | n/a | Larsson et al. (2017) |
| I. fibrosa (sowerby) Gillet | EL2599 | Estonia | AM882846 | AM882846 | n/a | Ryberg et al. (2008) |
| I. flavipes Aïgnon, Yorou & Ryberg | MR00383 | Togo | MN096197 | MN097889 | MW080915 | This study |
| HLA0363 | Benin | MT994601 | n/a | n/a | ||
| L4512_Inoc_Zam05 | Zambia | FR731552 | n/a | n/a | Tedersoo et al. (2011) | |
| I. flavoalbida Matheny & Bougher | PBM3768 | Australia | KJ729873 | KJ729901 | KJ729932 | Matheny & Bougher (2017) |
| I. flavobrunnescens Esteve-Rav., G. Moreno & Bizio | EL484-13 | Spain | MK153642 | MK153642 | n/a | Esteve-Raventós et al. (2015) |
| I. flavosquamulosa C.K. Pradeep & Matheny | CAL 1353 | India | KY440087 | KY549117 | KY553238 | Latha & Manimohan (2017) |
| I. flavosquamulosa C.K. Pradeep & Matheny | CAL 1355 | India | KY440088 | KY549118 | n/a | Latha & Manimohan (2017) |
| I. floccosistipitata K.P.D. Latha & Manim. | CAL 1256 | India | KY440089 | KY549119 | KY553239 | Latha & Manimohan (2017) |
| I. fuligineoatra Huijsman | PBM 2662 | USA | EU523589 | EU307831 | EU307833 | Matheny et al. (2009) |
| I. fuscata Singer | MES544 | Chile | KP171120 | KP170909 | KM245986 | Horak et al. (2015) |
| I. fuscicothurnata Grund & D.E. Stuntz | PBM3980 | USA | MF487844 | KY990485 | MF416408 | Larsson et al. (2014) |
| I. fuscobrunnea Aïgnon, Yorou & Ryberg | MR00378 | Burkina Faso | MN096201 | MN097893 | MW21933 | This study |
| HLA0567 | Ivory Coast | MT994603 | n/a | n/a | ||
| I. giacomi J. Favre | JV21543 | Finland | MK153656 | MK153656 | n/a | Cripps et al. (2019) |
| I. glaucodisca Buyck & Eyssart. | PC 96081 | Zambia | n/a | EU569853 | n/a | Matheny et al. (2009) |
| I. godeyi Gillet | JV 14914F | Italy | n/a | AY038316 | AY337378 | Matheny et al. (2002) |
| I. gracilior E. Horak | PDD:72707 | New Zealand | KY827277 | KY827242 | n/a | Horak (2018) |
| I. griseovelata Kühner | PBM2442 | USA | KC305453 | JN974938 | KC305420 | Ryberg & Matheny (2012), Braaten et al. (2014) |
| I. hirculus Vauras | JV30693 | Finland | MK153643 | MK153643 | n/a | Cripps et al. (2019) |
| I. horakomyces Garrido | PDD:72491 | New Zealand | KY827286 | KY827251 | n/a | Horak (2018) |
| I. humidicola Matheny & Bougher | PBM3719 | Australia | KP171126 | KJ801181 | KJ811575 | Matheny & Bougher (2017) |
| I. hydrocybiformis (Corner & E. Horak) Garrido | CAL 1376 | India | KY440090 | KY549120 | KY553240 | Latha & Manimohan (2017) |
| I. hystrix (Fr.) P. Karst. | SJ020824 | Sweden | AM882810 | AM882810 | n/a | Ryberg et al. (2008) |
| I. impexa (Lasch) Kuyper | TAA172127 | Finland | AM882821 | AM882821 | n/a | Ryberg et al. (2008) |
| I. insulana K.P.D. Latha & Manim. | CAL 1258 | India | KY440092 | KY549122 | KY553241 | Latha & Manimohan (2017) |
| I. iringolkavensis K.P.D. Latha & Manim. | K(M) 191731 | India | KM924524 | KM924519 | KY553242 | Latha & Manimohan (2017) |
| I. johannis-stanglii Bandini, Esteve-Rav. & G. Moreno | BAN959 KR M 0043321 | Germany | KX290793 | n/a | MH496019 | Esteve-Raventós et al. (2018) |
| I. kapila K.P.D. Latha & Manim. | CAL 1346 | India | KY440093 | KY549123 | n/a | Latha & Manimohan (2017) |
| I. kohistanensis Jabeen, I. Ahmad & Khalid | LAH 35001 | Pakistan | NR_153155 | n/a | n/a | Jabeen et al. (2015) |
| I. krieglsteineri Fern. Sas. | RFS031213-03 | France | KT958914 | KT958914 | n/a | Kropp et al. (2010) |
| I. kurkuriya K.P.D. Latha & Manim. | CAL 1352 | India | KY440095 | KY549125 | KY553245 | Latha & Manimohan (2017) |
| I. kuruvensis K.P.D. Latha & Manim. | K(M) 191734 | India | KM924522 | KM924517 | KY553246 | Latha & Manimohan (2017) |
| I. lacera (Fr.) P. Kumm. | PBM2541 | USA | KP171144 | JN974993 | KM245991 | Ryberg & Matheny (2012) |
| I. lacunarum Vauras & E. Larss. | JV12244 | Finland | KT958908 | KT958908 | n/a | Vauras & Larsson (2015) |
| I. lanuginosa (Bull.) P. Kumm | PBM3023/PBM3719 | USA | KP171126 | KP170923 | KM245992 | Matheny et al. (2020) |
| I. lasseri Dennis | MCA 1971 | Guyana | n/a | EU569857 | EU569856 | Matheny et al. (2009) |
| I. lasseroides (E. Horak) Garrido | PBM3749 | Australia | KP171145 | KP170924 | KM245993 | Horak et al. (2015) |
| I. leptospermi (E. Horak) Garrido | PBM3628 | New Zealand | KP308758 | KP170935 | KJ811592 | Matheny & Bougher (2017) |
| I. lilacinosquamosa Matheny, Aime & T.W. Henkel | MCA 1464 | Guyana | n/a | AY380386 | AY337389 | Matheny et al. (2009) |
| I. lineata E. Horak, Matheny & Desjardin | DED8048 | Thailand | n/a | GQ892958 | KM245999 | Horak et al. (2015) |
| I. luteifolia A.H. Sm., | PBM2642 | USA | n/a | EU307814 | EU307816 | Kropp et al. (2010) |
| I. luteo-olivacea Matheny, Bougher & Halling | NY:01491109 | Australia | KP308772 | KP170946 | KJ811603 | Matheny & Bougher (2017) |
| I. melanopoda D.E. Stuntz 1954 | PBM3975 | USA | n/a | MH220276 | MH249807 | Matheny et al. (2020) |
| I. mendica E. Horak | PDD:97864 | New Zealand | KP308780 | KP170951 | KM406193 | Horak et al. (2015) |
| I. mixtilis (Britzelm.) Sacc. | ARAN-Fungi 4711 | Spain | MH500842 | MH500842 | MH496022 | Esteve-Raventós et al. (2018) |
| I. murina E. Larss., C.L. Cripps & Vauras | CLC1226 | USA | MK153679 | MK153679 | n/a | Cripps et al. (2019) |
| I. muthangensis K.P.D. Latha & Manim. | K(M) 191735 | India | KM924521 | KM924516 | KY553247 | Latha & Manimohan (2017) |
| I. napipes J.E. Lange | PBM 2376 | Norway | n/a | AY239024 | AY337390 | Matheny (2005) |
| I. nothomixtilis Esteve-Rav., Bandini & V. González | AH 46558, MC0003 | Spain, Italy | MT384015 | n/a | MH496025 | Kropp & Matheny (2004) |
| I. oblectabilis (Britzelm.) Sacc. | BJ920908 | Sweden | AM882831 | AM882831 | n/a | Ryberg et al. (2008) |
| I. obtusiuscula Kühner | PAM02081710 | France | HQ586869 | HQ641112 | n/a | Matheny & Bougher (2017) |
| I. occulta Esteve-Rav., Bandini, B. Oertel & G. Moreno | AH 36443 | Spain | NR_160564 | n/a | MH496017 | Esteve-Raventós et al. (2018) |
| I. ohenojae Vauras & E. Larss. | ohenojae02081975 | Canada | KJ399955 | KJ399955 | n/a | Larsson et al. (2014) |
| I. olivaceohinnulea Matheny & Bougher | PBM3624 | Australia | KP308797 | KP170965 | KM406205 | Matheny & Bougher (2017) |
| I. pallidiangulata Aïgnon, Yorou & Ryberg | HLA0563 | Burkina Faso | MZ605435 | n/a | n/a | This study |
| MR00377 | Burkina Faso | MN096202 | MN097894 | MW21932 | ||
| MR00379 | Burkina Faso | MZ605434 | n/a | n/a | ||
| MR00384 | Burkina Faso | MZ605433 | n/a | n/a | ||
| I. pallidicremea Grund & D.E. Stuntz | PBM2448 | USA | HQ201357 | HQ201357 | MF416425 | Matheny et al. (2020) |
| I. papilliformis C.K. Pradeep & Matheny | CAL 1372 | India | KY440096 | KY549126 | n/a | Latha & Manimohan (2017) |
| I. paragiacomi E. Larss., C.L. Cripps & Vauras | EL64-11 | Sweden | MK153670 | MK153670 | n/a | Cripps et al. (2019) |
| I. parvibulbosa E. Horak, Matheny & Desjardin | SFSU:DED8021 | Thailand | GQ892999 | GQ892954 | KM555134 | Horak et al. (2015) |
| I. persicinipes Matheny & Bougher | PBM2197/E7044 | Australia | KF977215 | EU600837 | EU600836 | Matheny et al. (2009), Matheny & Bougher (2017) |
| I. phaeocystidiosa Esteve-Rav., G. Moreno & Bon, | CLC1133 | USA | MK153630 | MK153630 | n/a | Cripps et al. (2019) |
| I. phaeoleuca Kühner | EL297-08 | Hungary | KJ399958 | KJ399958 | n/a | Seress et al. (2016) |
| I. phaeosticta Furrer-Ziogas | PAM05091310 | France | HQ586859 | HQ641102 | MH577435 | Unpublished |
| I. pileosulcata E. Horak, Matheny & Desjardin | CAL 1362 | India | KY440098 | KY549128 | n/a | Latha & Manimohan (2017) |
| I. pileosulcata E. Horak, Matheny & Desjardin | CAL 1368 | India | KY440099 | KY549129 | n/a | Latha & Manimohan (2017) |
| I. pingala K.P.D. Latha & Manim. | CAL 1345 | India | KY440100 | KY549130 | KY553248 | Latha & Manimohan (2017) |
| I. pluppiana Bandini, B. Oertel & U. Eberh. | SMNS-STU-F-0901254 | Netherlands | MN512327 | MN512327 | n/a | Bandini et al. (2020a) |
| I. pluvialis Matheny, Bougher & G.M. Gates | PBM3228 | Australia | KF871777 | KF853401 | KF891954 | Matheny & Bougher (2017) |
| I. populea Takah. Kobay. & Courtec. | TAKK15655 | Japan | KT958911 | n/a | n/a | Kobayashi & Courtecuisse (2000) |
| Inocybe praetervisa Quél | AH44415 | Spain | KT203793 | KT203793 | n/a | Esteve-Raventós et al. (2015) |
| SF229598 | Italy | KT203792 | n/a | n/a | ||
| UBC:F19322 | Canada | HQ604397 | HQ604397 | n/a | ||
| UBC:F19334 | Canada | HQ604401 | HQ604401 | n/a | ||
| I. pseudodestricta Stangl & J. Veselský | JV061030 | Italy | FN550908 | FN550908 | n/a | Unpublished |
| I. pulchella Matheny, Aime & T.W. Henkel | MCA 1122 | Guyana | n/a | EU600842 | n/a | Matheny et al. (2009) |
| I. purpureobadia Esteve-Rav. & A. Caball. | CLC 1205 | USA | MK153689 | MK153689 | n/a | Cripps et al. (2019) |
| I. purpureoflavida K.B. Vrinda & C.K. Pradeep | CAL 1379 | India | KY440101 | KY549131 | n/a | Latha & Manimohan (2017) |
| I. rekhankitha K.P.D. Latha & Manim. | CAL 1356 | India | KY440102 | KY549132 | n/a | Latha & Manimohan (2017) |
| I. relicina (Fr.) Quél. | JV 10258 | Finland | n/a | AY038324 | AY333778 | Matheny et al. (2002) |
| I. rivularis Jacobsson & Vauras | Weholt120818 | Norway | KY033811 | KY033811 | n/a | Unpublished |
| I. roseifolia Murrill | CO5576 | USA | n/a | MK421968 | MH577441 | Matheny et al. (2020) |
| I. rufobadia Matheny & Bougher | NLB885 | Australia | KF977213 | KF915290 | KF991385 | Matheny & Bougher (2017) |
| I. sambucina (Fr.) Quél. | SJ01002 | Sweden | AM882757 | AM882757 | n/a | Ryberg et al. (2008) |
| I. scissa (E. Horak) Garrido | PDD:82757 | New Zealand | KY827287 | KY827239 | n/a | Horak (2018) |
| I. sejuncta Matheny, Bougher & M.D. Barrett | PBM3752 | Australia | n/a | KP171002 | KM555103 | Matheny & Bougher (2017) |
| I. serrata Cleland | PBM3235 | Australia | KP636810 | KP171012 | KM555111 | Matheny & Bougher (2017) |
| I. silvana K.P.D. Latha & Manim. | CAL 1259 | India | KY440104 | KY549134 | n/a | Latha & Manimohan (2017) |
| I. snigdha K.P.D. Latha & Manim. | CAL 1350 | India | KY440105 | KY549135 | KY553250 | Latha & Manimohan (2017) |
| I. soluta Velen. | EL2904 | Sweden | AM882755 | AM882755 | n/a | Ryberg et al. (2008) |
| Inocybe sp. | PC 96039 | Zambia | n/a | EU555474 | EU555473 | Matheny et al. (2009) |
| Inocybe sp. | PC 96095 | Zambia | n/a | EU569860 | n/a | Matheny et al. (2009) |
| Inocybe sp. | PC 96111 | Zambia | EU600875 | EU600875 | n/a | Matheny et al. (2009) |
| Inocybe sp. | MR00219 | Australia | KF830031 | KF808343 | KF830049 | Matheny & Bougher (2017) |
| Inocybe sp. | PERTH:08383235 | Australia | KP636839 | KP171043 | KM555138 | Matheny & Bougher (2017) |
| Inocybe sp. | 3243F7 | USA | KF618024 | KF618024 | n/a | Unpublished |
| Inocybe sp. | DPL12128 | USA | MH578022 | MT241843 | MH618233 | Unpublished |
| Inocybe sp. | D25 (WTU) | Argentina | n/a | AY380363 | AY337361 | Matheny (2005) |
| Inocybe sp. | L4517e_Inoc_Zam11 | Zambia | FR731548 | n/a | n/a | Tedersoo et al. (2011) |
| Inocybe sp. | 96083 (PC) | Zambia | n/a | EU600884 | n/a | Matheny et al. (2009) |
| Inocybe sp. | ZT10031 (SFSU) | Thailand | GQ893020 | GQ892976 | n/a | Horak et al. (2015) |
| I. spadicea Matheny & Bougher | PBM2203 | Australia | KP636866 | EU600865 | n/a | Matheny et al. (2009), Matheny & Bougher (2017) |
| I. spiniformis Matheny & Bougher | PBM3748 | Australia | KP636868 | KP171064 | KM656103 | Matheny & Bougher (2017) |
| I. splendens R. Heim | EL313-12 | France | KJ399959 | KJ399959 | n/a | Larsson et al. (2014) |
| I. stellata E. Horak, Matheny & Desjardin | CAL 1369 | India | KY440106 | KY549136 | KY553251 | Latha & Manimohan (2017) |
| I. strickeriana Bandini, Anja Schneid. & M. Scholler | KR:KR-M-0044749 | Germany | MG012477 | MG551670 | n/a | Bandini et al. (2018) |
| I. subcarpta Kühner & Boursier | EL8905 | Finland | AM882754 | AM882754 | n/a | Ryberg et al. (2008) |
| I. subexilis (Peck) Sacc. | PBM2620/ACAD:11680 | USA/Canada | MH578001 | EU307845 | EU307847 | Esteve-Raventós et al. (2018) |
| I. subferruginea Matheny & Bougher | E7066 (PERTH) | Australia | n/a | EU600894 | n/a | Matheny et al. (2009) |
| I. subfibrosoides Singer | FLAS:MES543 | Chile | KP636879 | KP171073 | KM656117 | Matheny & Bougher (2017) |
| I. subfulva Peck | PBM1482 | USA | KP641623 | JN974989 | n/a | Ryberg & Matheny (2012), Matheny & Bougher (2017) |
| I. subgiacomi C.L. Cripps, Vauras & E. Larss | JV29938 | Sweden | MK153665 | MK153665 | n/a | Cripps et al. (2019) |
| I. subporospora Kuyper | RP950618 | Sweden | AM882931 | AM882931 | n/a | Ryberg et al. (2008) |
| I. substellata Kühner | EL52-13 | France | KT958927 | KT958927 | n/a | Vauras & Larsson (2016) |
| I. subtrivialis Esteve-Rav., M. Villarreal & Heykoop | AH26789 | Spain | KX354977 | n/a | MH496024 | Esteve-Raventós et al. (2018) |
| I. sylvicola Matheny, Bougher & G.M. Gates | TENN:065735 | Australia | NR_153163 | NG 057199 | KM656126 | Horak et al. (2015) |
| I. taxocystis (J. Favre & E. Horak) Senn-Irlet | Voucher358 | Italy | JF908095 | n/a | n/a | Osmundson et al. (2013) |
| I. tertia Matheny & Bougher | PBM3730, TENN:066951 | Australia | KP641633 | KP171086 | KM656128 | Matheny & Bougher (2017) |
| I. thailandica E. Horak, Matheny & Desjardin | DED8049 | Thailand | GQ893013 | GQ892968 | KM656129 | Matheny et al. (2009), Horak et al. (2015) |
| I. torresiae Matheny, Bougher & M.D. Barrett | PBM2157/E6978 | Australia | KP641635 | EU600874 | EU600873 | Matheny et al. (2009), Horak et al. (2015) |
| I. tubarioides G.F. Atk. | PBM 2570 | USA | n/a | AY732210 | n/a | Matheny & Moreau (2009) |
| I. tumidula Matheny & Bougher | PBM3770 | Australia | KP641638 | KP171090 | KM656134 | Horak et al. (2015), Matheny & Bougher (2017) |
| I. turbata E. Horak | PDD:82818 | New Zealand | KY827281 | KY827245 | n/a | Horak (2018) |
| I. umbrosa E. Horak | PDD:106098 | New Zealand | MN047364 | MN047420 | n/a | Unpublished |
| I. undinea Bandini, P.-A. Moreau, B. Oertel | FR-0246019 | Germany | MK929265 | n/a | n/a | Bandini et al. (2020b) |
| I. villosa Bandini, B. Oertel & U. Eberh. | BAN1420 | Germany | MG012478 | n/a | n/a | Esteve-Raventós et al. (2018) |
| I. viraktha K.P.D. Latha & Manim. | CAL 1357 | India | KY440107 | KY549137 | KY553252 | Latha & Manimohan (2017) |
| I. vulpinella Bruyl. | NI250904 | Canada | n/a | EU307834 | n/a | Matheny et al. (2009) |
| I. wayanadensis K.P.D. Latha & Manim. | K(M) 191737 | India | KM924520 | KM924515 | KY553254 | Latha & Manimohan (2017) |
| I. xanthomelas Boursier & Kühner | PAM08082901 | France | HQ586856 | HQ641097 | n/a | Esteve-Raventós et al. (2015) |
| I. xerophytica Pegler | GUA242 | British Virgin Islands | n/a | EU600880 | n/a | Matheny et al. (2009) |
| Nothocybe distincta (K.P.D. Latha & Manim.) Matheny & K.P.D. Latha, | CAL 1310 | India | KX171343 | NG_057278 | KX171345 | Matheny et al. (2009), Latha et al. (2016) |
| ZT 9250 | India | n/a | EU604546 | EU600904 | ||
Fig. 1.

ML tree of ITS, 28S and RPB2 sequences showing the placement of four new species described from tropical regions of Africa: Inocybe beninensis, I. flavipes I. fuscobrunnea and I. pallidiangulata. Filled circles indicate internodes that are strongly supported (bootstrap proportions SHaLRT support ≥ 80 % / ultrafast bootstrap support ≥ 95 % / Bayesian posterior probabilities > 0.95). Empty circles indicate internodes with moderate support where at least one, but not all, support statistics meet the criteria for strong support). The geographic origin of each tip is given after the species name.
Inocybe beninensis and I. flavipes were part of a subclade leading to the I. calida group, together with the specimen Inocybe sp. L4517e_Inoc_Zam11 from Zambia (belonging to what seems to be an undescribed species). Inocybe beninensis in turn was weakly supported (60 % SH-aLRT values, 75 % ML ultrafast bootstrap, 0.8 BPP) as sister to the common clade that they formed. The low sequence divergences (1.9 %) between the sequences of ITS of the collections MR00383, HLA0363, investigated here, and the sequence of the collection L4512_Inoc_Zam05 from Zambia, indicated that they may very well be conspecific, suggesting a wide distribution for I. flavipes.
Inocybe fuscobrunnea was moderately supported (88 % SH-aLRT values, 50 % ML ultrafast bootstrap, 0.6 BPP) as sister to the I. asterospora group including the European species I. fibrosa and I. asterospora, and Asian species I. rekhankitha, I. silvana and I. pileosulcata.
Inocybe pallidiangulata was weakly supported (54 % SH-aLRT values, 60 % ML ultrafast bootstrap, 0.7 BPP) as member of an inclusive clade with a major tropical component; including many species from India, Zambia, and Guyana.
Taxonomy
Inocybe beninensis Aïgnon, Yorou & Ryberg, sp. nov. MycoBank MB 837971. Figs 2, 3, 10A, B.
Fig. 2.

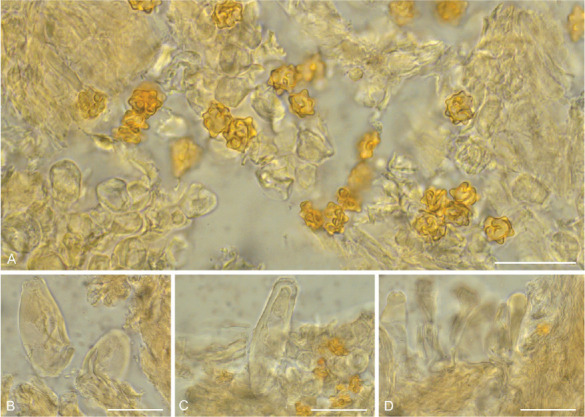
Micromorphology of Inocybe beninensis (HLA0390). A. Basidio-spores. B. Basidia. C. Cheilocystidia. D. Pleurocystidia. E. Caulocystidia. F. Pileipellis. G. Stipitipellis. Scale bars: A = 3 μm, B = 5 μm; C–G = 10 μm.
Fig. 3.

Inocybe beninensis (HLA0390), microscopical characters in KOH. A. Basidiospores. B. Cheilocystidia. C. Pleurocystidia. D. Caulocystidia. Scale bars = 20 μm.
Fig. 10.

Macro-morphology. A, B. Inocybe beninensis (A = HLA0390, B = HLA0462). C. Inocybe flavipes (MR00383). D. Inocybe fuscobrunnea (MR00378). E, F. Inocybe pallidiangulata (E = MR00377, F = HLA0565). Bar = 1 cm.
Etymology: beninensis (L.), referring to the type locality of Benin.
Diagnosis: Inocybe beninensis is morphologically similar to I. flavipes from Benin and Togo but differs from it by the larger basidiospores on average (10.4 × 8.8 μm vs. 8.6 × 5.3 μm) and whitish yellow stipe.
Typus: Benin, Collines region, Toui-Kilibo forest reserve, 8.545722N, 2.67375E, on soil in woodlands dominated by Isoberlinia doka and I. tomentosa, 22 Jun. 2017, leg. H.L. Aïgnon (holotype HLA0390, deposited at UNIPAR). GenBank accessions: ITS (MN096196) and 28S (MN097888).
Description: Pileus 7–20 mm wide, conical to plane, sometimes umbonate, fibrillose to tomentose, yellowish (oac715) to orange-brown (oac719); margin dentate. Flesh white, 2 mm thick at umbo, wider towards the edges, margin smooth. Lamellae 2–4 mm deep, regular, adnexed to adnate, moderately close, white when young becoming yellowish orange (oac768) with age, edge slightly crisped. Lamellulae multi-tiered. Stipe 18–20 × 1.5–3 mm, hollow, flocculose, yellowish white (oac717), central, cylindrical, straight or curved, pruinose, surface slightly fibrillose at the base, veil present. Odour and taste not distinctive. Basidiospores (8.0–)8.7–12.0(–12.1) × (6.0–)6.6–10.9(–11.0) μm, avl × avw = 10.4 × 8.8 μm, Q = (1–)0.9–1.4(–1.5), avQ = 1.1, globose, distinctly nodulose with mostly 4–6 conic nodules, brown in KOH under the microscope. Basidia 27–40 × 9–12 μm, 4–spored, clavate to cylindric. Cheilocystidia 24–55 × 11–18 μm, subclavate, sometimes thin-walled, with apex sometimes crystalliferous. Pleurocystidia 32–44 × 10–15 μm, utriform, thick-walled. Caulocystidia 42–45 × 11–25 μm, pyriform to clavate, thin-walled, observed only at the apex of the stipe. Pileipellis a trichoderm of filamentous hyphae; subpellis of compact hyphae 3–8 μm wide, thin-walled. Stipitipellis a cutis of subcylindrical hyphae 5–20 μm wide, hyaline, thick-walled, with differentiated terminal cells.
Habit: Solitary or in groups, scattered on soil.
Habitat: In woodland dominated by Isoberlinia doka and I. tomentosa. Occurring June to September.
Geographical distribution: Hitherto known from Benin only.
Additional specimen examined: Benin, Borgou Province, N’dali forest reserve, 9.74279N, 2.6929277E, on soil in woodlands dominated by Isoberlinia doka and I. tomentosa, 1 Sep. 2017, leg. H.L. Aïgnon, Specimen voucher (HLA0467). GenBank accession: ITS (MT994602).
Inocybe flavipes Aïgnon, Yorou & Ryberg, sp. nov. MycoBank MB 837975. Figs 4, 5, 10C.
Fig. 4.
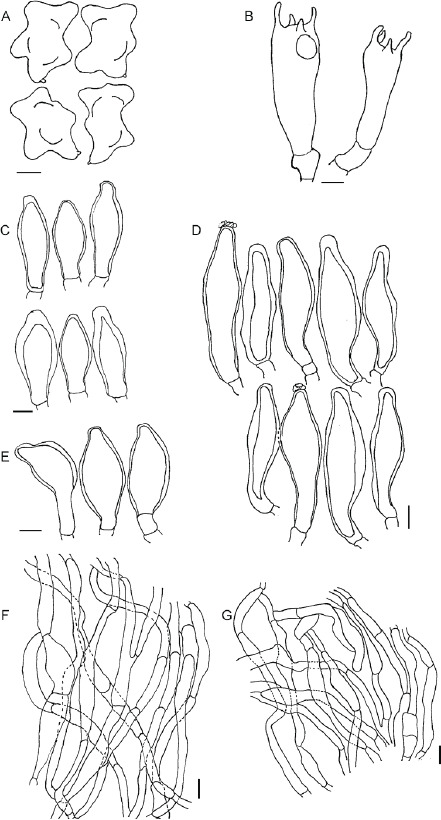
Micromorphology of Inocybe flavipes (MR00383). A. Basidio-spores. B. Basidia. C. Cheilocystidia. D. Pleurocystidia. E. Caulocystidia. F. Pileipellis. G. Stipitipellis. Scale bars: A = 3 μm; B = 5 μm; C–G = 10 μm.
Fig. 5.

Inocybe flavipes (MR00383), microscopical characters in KOH. A. Basidiospores. B. Cheilocystidia. C. Pleurocystidia. D. Caulocystidia. Scale bars: A–D = 20 μm.
Etymology: flavipes (L.), referring to the yellow stipe.
Diagnosis: Inocybe flavipes is most similar to I. beninensis from Benin but differs from it by the absence of a veil on the stipe, stipe light yellow to light orange, and smaller basidiospores on average (8.6 × 5.3 μm vs. 10.4 × 8.8 μm).
Typus: Togo, Central region, prefecture of Assoli, Aledjo forest reserve, 9.340278N, 1.251944E, on soil in gallery forest dominated by Isoberlinia tomentosa, 17 Jul. 2013, leg. M. Ryberg (holotype MR00383, deposited at UNIPAR). GenBank accessions: ITS (MN096201), 28S (MN097893) and RPB2 (MW080915).
Description: Pileus 6–13 mm wide, hemispherical, conical to conical with broad umbo, surface fibrillose to minutely scaly, yellow brown (oac734) to orange-brown (oac755). Flesh white, 1mm thick at umbo, margin smooth. Lamellae 2.5 mm deep, adnexed, lamellulae in tiers, brown (oac734). Stipe 14–24 × 1–1.5 mm, surface fibrillose, pruinose in the upper part, more or less equal but with a basal rounded bulb, whitish remnants of a velipellis in lower parts, light yellow (oac716) to light orange (oac718). Odour and taste not distinctive. Basidiospores (6–) 6.3–10(–10.9) × (3.7–)4–10(–10.4) μm, avl × avw = 8.6 × 5.3 μm, Q = (1–)1.1–2.2(2.5) μm, avQ = 1.6, elongate, with 3–5, obtuse nodules, prominent and distinct. Basidia 20–32 × 6–10 μm, generally 4–spored, sometimes 2 or 3-spored. Cheilocystidia 32–51 × 11–22 μm, obovoid, apex sometimes crystalliferous, thick-walled. Pleurocystidia 35–60 × 12–22 μm, utriform, sometimes obovoid, with short pedicel, sometimes with rounded or truncate base, thick-walled, apex usually crystalliferous. Caulocystidia 23–45 × 10–22 μm, sometimes crystalliferous, in upper part of the stipe only, pale brown apex usually. Pileipellis a cutis made up of subparallel hyphae 3–12 μm wide. Stipitipellis a trichoderm of dense, compact hyphae 4–12 μm wide and thick-walled.
Habit: Solitary, in pairs or in small groups, scattered on soil.
Habitat: In gallery forest dominated by Isoberlinia tomentosa or Isoberlina doka. Occurring in June to July.
Geographical distribution: Until now known from West Africa: Benin and Togo.
Additional specimen examined: Benin, Borgou Province, Commune of Tchaourou, 9.2446277 N, 2.7262333E, on soil in Okpara forests dominated by Isoberlina doka, 10 Jun. 2017, leg. H.L. Aïgnon, Specimen voucher (HLA0363), GenBank accession: ITS (MT994601).
Inocybe fuscobrunnea Aïgnon, Yorou & Ryberg, sp. nov. MycoBank MB 837976. Figs 6, 7, 10D.
Fig. 6.

Micromorphology of Inocybe fuscobrunnea (MR00378). A. Basidiospores. B. Basidia. C. Cheilocystidia. D. Pleurocystidia. E. Caulocystidia. F. Pileipellis. G. Stipitipellis. Scale bars: A = 3 μm, B = 4 μm, C–G = 10 μm.
Fig. 7.
Inocybe fuscobrunnea (MR00378), microscopical characters in KOH. A. Basidiospores. B. Cheilocystidia. C. Pleurocystidia. D. Caulocystidia. Scale bars: A–D = 20 μm.
Etymology: fuscobrunnea (L.), referring to the dark brown pileus.
Diagnosis: Inocybe fuscobrunnea has a dark brown, slightly rimose pileus, nodulose spores measuring 7–12 × 6–10.4 μm, similar to I. pallidiangulata in size, but differs from it by the dark reddish brown pileus and larger basidiospores (9.5 × 8.3 μm vs. 7.0 × 5.2 μm).
Typus: Burkina Faso, Bobo-Dioulasso region, forest reserve of Kou, 11.188027N, 4.440556W, on soil in gallery forests dominated by Berlinia grandiflora, 13 Jul. 2013, leg. M. Ryberg (holotype MR00378, deposited at UNIPAR). GenBank accessions: ITS (MN096201), 28S (MN097893) and RPB2 (MW21933).
Description: Pileus 19–40 mm wide, wide conical to plane with obtuse umbo, surface flocculose/tomentose to smooth rimose, brown (oac646) to dark reddish brown (oac836-838), margin smooth, slightly rimose. Flesh white, 2 mm thick under the disc, thinner towards edges, margin rimose. Lamellae 3–5 mm deep, adnexed, pale grey (oac809) turning brown with age (oac807), 34 reaching stipe. Lamellae alternate with lamellulae, multi-tiered. Stipe 32–72 × 2.5–4 mm, subclavate, mostly silky greyish yellowish white (oac857), smooth to longitudinally substriate, pruinose in the upper 1/3 part. Odour and taste not distinctive. Basidiospores (7–)7.6–11.4(–12) × (6–)6.2–10(–10.4) μm, avl × avw = 9.5 × 8.3 μm, Q = 0.9–1.4 μm, avQ = 1.1, globose, with 3–6 obtuse nodules. Basidia 25–38 × 9–14 μm, ventricose, 4-spored. Cheilocystidia 30–47 × 12–20 μm, utriform sometimes obovoid, hyaline crystals on the top, often short pedicels, thin-walled. Pleurocystidia 35–52 × 11–27 μm, utriform, apex sometimes crystalliferous, rounded or truncate base, thin-walled. Caulocystidia 38–51 × 12–17 μm, thin-walled, sometimes crystalliferous, observed only near of the stipe’s apex. Pileipellis a cutis composed of parallel hyphae 4–31 μm wide. Stipitipellis a cutis of subparallel hyphae 6–17 μm wide, hyaline and thick-walled.
Habit: Solitary or in small groups, scattered on soil.
Habitat: In gallery forest dominated by Berlinia grandiflora. Occurring in July.
Geographical distribution: Known from West Africa: Burkina Faso and Ivory Coast.
Additional specimen examined: Ivory Coast, Koudianikro, gbeke region, Bouake, 7.629444N, 4.746667W, on soil in woodland dominated by Berlinia grandiflora, 11 Jul. 2018, leg. L.H. Aïgnon, Specimen voucher (HLA0567), deposited at UNIPAR. GenBank accession: ITS (MT994603).
Inocybe pallidiangulata Aïgnon, Yorou & Ryberg, sp. nov. MycoBank MB 837977. Figs 8, 9, 10E, F.
Fig. 8.

Fig. 8. Micromorphology of Inocybe pallidiangulata (MR00377). A. Basidiospores. B. Basidia. C. Cheilocystidia. D. Pleurocystidia. E. Caulocystidia. F. Pileipellis. G. Stipitipellis. Scale bars: A = 3 μm, B = 5 μm; C–G = 10 μm.
Fig. 9.
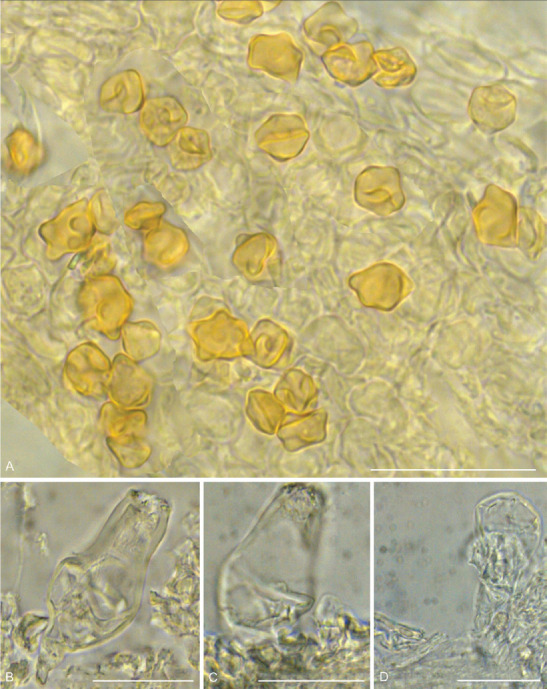
Inocybe pallidiangulata (MR00377), microscopical characters in KOH. A. Basidiospores. B. Cheilocystidia. C. Pleurocystidia. D. Caulocystidia. Scale bars: A–D = 20 μm.
Etymology: pallidiangulata (L.), referring to the pale pileus and angular spores.
Diagnosis: Inocybe pallidiangulata is characterised by a pale brown to honey-yellow pileus, nodulose spores measuring 6–9 × 3–6.4 μm, caulocystidia only at the apex of the stipe, and apparent ectomycorrhizal association with tropical Fabaceae and/or Phyllanthaceae. It is superficially similar to I. fuscobrunea by being rimose pileus, similar in size, and occurring in the same habitat but differs from it by the paler pileus and smaller basidiospores with very inconspicuously protruding nodules.
Typus: Burkina Faso, Bobo Dioulasso, forest reserve of Kou, 11.186861N, 4.4415277W, on soil in gallery forest dominated by Berlinia grandiflora. 17 Jul. 2013, leg. M. Ryberg (holotype MR00377, deposited at UNIPAR). GenBank accession: ITS (MN096202), 28S (MN097894) and RPB2 (MW21932).
Description: Pileus 11–29 mm wide, oval, obtuse, conical when young, becoming plane with age, umbo wide, surface fibrillose, fibrils, pale brown (oac825), almost honey-yellow (oac826). Flesh white to greyish white (oac847), 5 mm thick at centre, thinner at edges, margins serrate and slightly rimose. Lamellae 3 mm deep, adnexed, 36 reaching stipe, grey white (oac816). Lamellulae multi-tiered. Stipe 14–45 × 4.5–6 mm, equal to slightly wider at the centre, base bulbous having marginate bulb in young specimens, not obvious in old specimens, stipe surface longitudinally striated, white when young becoming yellow (oac858) with age, possible remnants of veil. Odour and taste not distinctive. Basidiospores (6–)5.6 –8.5(–9) × (3–) 4–6(–6.4) μm, avl × avw = 7.0 × 5.2 μm, Q = (1–)1.1–1.7(–1.8) μm, avQ = 1.4, ellipsoid with 2–3, very inconspicuously protruding nodules. Basidia 20–35 × 7–12 μm, generally 4–spored, seldom 2–spored, clavate. Cheilocystidia 25–52 × 11–25 μm, utriform, truncate base or sometimes rounded, apex usually crystalliferous. Pleurocystidia 33–63 × 12–22 μm, obovoid sometimes utriform with long pedicel truncate base, apex usually crystalliferous wall thickness. Caulocystidia 35–40 × 16–27 μm obovoid, present at stipe apex only, pale brown apex sometimes crystalliferous. Pileipellis a cutis made up of compact hyphae, subparallel 4–12 μm wide. Stipitipellis a cutis often disrupted, composed of parallel hyphae 6–15 μm wide, thick-walled, encrusted, brownish pigment.
Habit: Solitary on soil.
Habitat: In gallery forest dominated by Berlinia grandiflora. Occurring in July.
Geographical distribution: West Africa: Burkina Faso, Ivory Coast and Togo.
Additional specimens examined: Burkina Faso, Bobo-Dioulasso region, forest reserve of Kou, 11.18694N, 4.441667W, on soil in gallery forest dominated by Berlinia grandiflora, 13 Jul. 2013, leg. M. Ryberg, specimen voucher (MR00379), deposited at UNIPAR. Ivory Coast, Kekrekouakoukro, Gbeke, Bouake region, 7.675388N, 4.908111W, on soil in woodland dominated by Berlinia grandfifolia 11 Jul. 2018, leg. H.L. Aïgnon, specimen voucher (HLA0565). Togo, Central region, prefecture of Assoli, forest reserve of Aledjo, 9.276944N, 1.225556E, on soil in gallery forest dominated by Berlinia grandiflora and Uapaca guineensis, 17 Jul. 2013, leg. M. Ryberg, specimen voucher (MR00384).
Taxonomic key to nodulose-spored Inocybe species from tropical Africa
1a. Basidiomata blackish blue and becoming green with a stipe slightly thickened at the base, basidiospores subglobose-quandrangular, 10–13 × 9–12 um, from Tanzania ......................................................................................................... Inocybe cyaneovirescens
1b. Basidiomata not blackish blue and not turning green, stipe base bulbous or not, basidiospores smaller than above if similar in size then basidiomata not turning green .......................................................................................................................................... 2
2a. Stipe pruinose along entire length .................................................................................................................................................... 3
2b. Stipe pruinose over the upper part or apex only ............................................................................................................................. 4
3a. Stipe robust with a marginate bulb, pale brown to subconcolourous with the pileipellis ........................................... I. glaucodisca
3b. Stipe remarkably slender, base not bulbous, dark brown but paler and more or less honey-brown in the upper part, with some reddish-brown tinges towards the base .......................................................................................................... I. conspicuospora
4a. Pileus margin rimose, straight, stipe surface longitudinally silky-striate, lacking smell when fresh .............................. I. ghanaensis
4b. Pileus margin not rimose, stipe surface not silky-striate, smell present when fresh ........................................................................ 5
5a. Pileus margin dentate or serrate ....................................................................................................................................................... 6
5b. Pileus margin smooth ........................................................................................................................................................................ 7
6a. Pileus fibrillose to tomentose, margins dentate, stipe hollow, flocculose, yellowish white, surface slightly fibrillose at the base, caulocystidia pyriform to clavate ............................................................................................................................. I. beninensis
6b. Pileus conical to conical with broad umbo, surface fibrillose to minutely scaly, stipe surface fibrillose, more or less equal but with a bulb, light yellow to light orange, caulocystidia crystalliferous .................................................................................... I. flavipes
7a. Stipe wider, white to yellow, base bulbous having marginate bulb in young specimens ........................................ I. pallidiangulata
7b. Stipe slender, mostly silky greyish yellowish white, base not bulbous ....................................................................... I. fuscobrunnea
DISCUSSION
Four species with nodulose-spores; Inocybe conspicuospora, I. cyaneovirescens, I. ghanaensis and I. glaucodisca have been described from tropical Africa before this study (Hennings 1902, Pegler 1969, Buyck & Eyssartier 1999), but many undescribed species with nodulose-spores are known from Zambia (Matheny et al. 2009, Tedersoo et al. 2011). Most of these species have a restricted distribution and so that what is found in tropical Africa is not likely to be the same species as something from other parts of the world (Buyck & Eyssartier 1999). Here we describe four additional new species of Inocybe; I. beninensis, I. flavipes, I. fuscobrunnea and I. pallidiangulata from tropical African regions. Our phylogenetic analyses show that I. conspicuospora and I. glaucodisca are distinct from the species described here by their distinct positions in the phylogenetic tree. Inocybe cyaneovirescens is distinguished by its colour and I. ghanaensis differs from the others by the presence of a marginate bulb. In the phylogenetic tree, as none of the new describe species belong in the I. praetervisa group, I. mixtilis group, I. napipes group, I. oblectabilis group, I. xanthomelas group, I. diabolica group, I. calospora group, I. lacera group, I. giacomi group, Smooth-spored temperate boreal clade and Smooth-spored temperate Austral clade, these groups or clades have been reduced without violating the result (Fig. 1). While all the species of these groups used in the phylogenetic analyses are shown in Supplementary Fig. S3. The new species described here emerge as sister to other groups, either by themselves or together with undescribed species from Zambia, or as even more isolated in the phylogeny (Fig.1).
The autonomy of the new species is supported by morphological and molecular data. The new species are currently known from Benin, Burkina Faso, Ivory Coast, Togo and/or Zambia and are associated with Isoberlinia doka, I. tomentosa, Berlinia grandiflora and/or Uapaca guineensis in West Africa and associated with the species of the family Phyllanthaceae and/or Fabaceae in miombo woodlands. All species described here have pleurocystidia with crystals and nodulose basidiospores, typical characteristics that support their placement in Inocybe (Matheny et al. 2020).
This study doubles the described diversity of the nodulose-spored species from tropical Africa, but we expect the actual number of species of Inocybe from Africa to be higher given the extent and diversity of ectomycorrhizal habitats, ectomycorrhizal tree species, and paucity of previous studies in this group.
ACKNOWLEDGEMENTS
We are grateful to our colleagues Kassim Tchan and Evans Codjia (Research Unit Tropical Mycology and Plant-Soil Fungi Interactions, University of Parakou) for their assistance during fieldwork and Anneli Svanholm, Bobby Sulistyo with Brandan Furneaux (Systematic Biology program, Department of Organismal Biology, Uppsala University) for their assistance during DNA extraction. Financial support for fieldwork was received from the National Geographic Society (grant n° CP 126R-17). The molecular analysis was supported by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (grant n° 226-2014-1109). We are also indebted to the Deutscher Akademischer Austauschdienst (DAAD, grant n° PKZ 300499) for granting the university of Parakou with a Leica DM5700 microscope that enabled us to perform microscopic investigations. Matheny was supported by a U.S. National Science Foundation grant (DEB-2030779). Yorou NS is grateful to the Federal Ministry for Education and Research (BMBF, Germany, grant No. 01D20015).
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Supplementary Material: http://fuse-journal.org/
ML tree of ITS+28S sequences showing the placement of Inocybe beninensis, I. flavipes I. fuscobrunnea and I. pallidiangulata. For each node, the ML ultrafast bootstrap support ≥ 95 % is presented above or in front of the branch leading to that node.
ML tree of RPB2 sequences showing the placement of Inocybe flavipes, I. fuscobrunnea and I. pallidiangulata. For each node, the ML ultrafast bootstrap support ≥ 95 % is presented above or in front of the branch leading to that node.
ML tree showing the placement of four new species described from tropical regions of Africa: Inocybe beninensis, I. flavipes I. fuscobrunnea and I. pallidiangulata based on phylogenetic analyses of ITS, 28S and RPB2 data set.
REFERENCES
- Aïgnon HL, Jabeen S, Naseer A, et al. (2021a). Three new species of Inosperma (Agaricales, Inocybaceae) from Tropical Africa. MycoKeys 77: 97–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aïgnon HL, Acar I, Naseer A, et al. (2021b). State of knowledge on the diversity, phylogeny and distribution of Inocybaceae in Africa. Asian Journal of Mycology 4: 56–66. [Google Scholar]
- Aïgnon HL, Naseer A, Matheny BP, et al. (2021c). Mallocybe africana (Inocybaceae, Fungi), the first species of Mallocybe described from Africa. Phytotaxa 478: 49–60. [Google Scholar]
- Akil ME, Khouader M, Chliyeh M, et al. (2015). A new species of Basidiomycetes for the fungal diversity of Morocco: Inocybe squarrosa and I. rufuloides. International Journal of Pure & Applied Bioscience 3: 27–34. [Google Scholar]
- Bandini D, Oertel B. (2020). Three new species of the genus Pseudosperma (Inocybaceae). Czech Mycology 72: 221–250. [Google Scholar]
- Bandini D, Oertel B, Moreau PA, et al. (2019). Three new hygrophilous species of Inocybe, subgenus Inocybe. Mycological Progress 18: 1101–1119. [Google Scholar]
- Bandini D, Oertel B, Ploch S, et al. (2018). Revision of some central European species of Inocybe (Fr.: Fr.) Fr. subgenus Inocybe, with the description of five new species. Mycological Progress 18: 247–294. [Google Scholar]
- Bandini D, Oertel B, Schüßler C, et al. (2020a). Noch Mehr Risspilze: Fünfzehn Neue Und Zwei Wenig Bekannte Arten Der Gattung Inocybe. Mycologia Bavarica 20: 13–101. [Google Scholar]
- Bandini D, Sesli E, Oertel B, et al. (2020b). Inocybe antoniniana, a new species of Inocybe section Marginatae with nodulose spores. Sydowia 72: 095–106. [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, et al. (2010). GenBank. Nucleic Acids Research 38: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon M. (1997). Clé monographique du genre Inocybe (Fr.) Fr. Documents Mycologiques 27: 1–51. [Google Scholar]
- Bon M. (1998). Clé monographique du genre Inocybe (Fr.) Fr. (3ème partie: espèces gibbosporèes, = g. Clypeus Britz. 5 genre Astrosporina Schroet.). Documents Mycologiques 28: 1–45. [Google Scholar]
- Braaten CC, Matheny PB, Viess DL, et al. (2014). Two new species of Inocybe from Australia and North America that include novel secotioid forms. Botany 92: 9–22. [Google Scholar]
- Buyck B, Eyssartier G. (1999). Two new species of Inocybe (Cortinariaceae) from African woodland. Kew Bulletin 54: 675–681. [Google Scholar]
- Caiafa MV, Sandoval-Leiva P, Matheny PB, et al. (2021). Four new species of sequestrate Inocybe from Chilean Nothofagaceae forests. Mycologia 113: 629–642. [DOI] [PubMed] [Google Scholar]
- Cripps CL, Larsson E, Vauras J. (2019). Nodulose-spored Inocybe from the Rocky Mountain alpine zone molecularly linked to European and type specimens. Mycologia 112: 133–153. [DOI] [PubMed] [Google Scholar]
- Cubeta M, Echandi E, Albernethy T. (1991). Characterization of anastomosis groups of binucleate Rhizoctonia species using restriction analysis of an amplified ribosomal RNA gene. Phytopathology 81: 1395–1400. [Google Scholar]
- Esteve-Raventós F, Alvarado P, Olariaga I. (2016). Unravelling the Inocybe praetervisa group through type studies and ITS data: Inocybe praetervisoides sp. nov. from the Mediterranean region. Mycologia 108: 123–134. [DOI] [PubMed] [Google Scholar]
- Esteve-Raventós F, Bandini D, Oertel B, et al. (2018). Advances in the knowledge of the Inocybe mixtilis group (Inocybaceae, Agaricomycetes), through molecular and morphological studies. Persoonia 41: 213–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve-Raventós F, Moreno G, Bizio E, et al. (2015). Inocybe flavobrunnescens, a new species in section Marginatae. Mycological Progress 14: 1–12. [Google Scholar]
- Gardes M, Bruns T. (1993). ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Hennings P. (1902). Fungi camerunenses novi. III. Botanische Jahrbücher fur Systematik, Pflanzengeschichte und Pflanzengeographie. 30: 39–57. [Google Scholar]
- Hoang DT, Chernomor O, von Haeseler A, et al. (2017). UFBoot2: Improving the Ultrafast Bootstrap Approximation. Molecular Biology and Evolution 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak E. (2018). Fungi of New Zealand/Ngâ Hekaheka o Aotearoa. Vol. 6. Agaricales (Basidiomycota) of New Zealand. 2. Brown spored gener p.p. Crepidotus, Flammulaster, Inocybe, Phaeocollybia, Phaeomarasmius, Pleuroflammula, Pyrrhoglossum, Simocybe, Tubaria and Tympanella. Utrecht, The Netherlands. [Westerdijk Biodiversity Series 16: 1–205]. Westerdijk Fungal Biodiversity Institute, Netherlands. [Google Scholar]
- Horak E, Matheny PB, Desjardin DE, et al. (2015). The genus Inocybe (Inocybaceae, Agaricales, Basidiomycota) in Thailand and Malaysia. Phytotaxa 230: 201–238. [Google Scholar]
- Jabeen S, Ahmad I, Rashid A, et al. (2015). Inocybe kohistanensis, a new species from Swat, Pakistan. Turkish Journal of Botany 40: 312–318. [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Courtecuisse R. (2000). Two new species of Inocybe, section Marginatae (Agaricales, Cortinariaceae) from Japan. Mycoscience 41: 161–166. [Google Scholar]
- Kropp BR, Matheny PB. (2004). Basidiospore homoplasy and variation in the Inocybe chelanensis group in North America. Mycologia 96: 295–309. [PubMed] [Google Scholar]
- Kropp BR, Matheny PB, Nanagyulyan SG. (2010). Phylogenetic taxonomy of the Inocybe splendens group and evolution of supersection “Marginatae”. Mycologia 102: 560–573. [DOI] [PubMed] [Google Scholar]
- Kuo M, Matheny PB. (2015). Contemporary documentation of the rare eastern North American species Inocybe insignis (Inocybaceae, Agaricales). MycoKeys 11: 23–31. [Google Scholar]
- Kuyper TWM. (1986). Subgenus Inosperma and the smooth-spored species of subgenus Inocybe. Persoonia Suppl. 3: 1–247. [Google Scholar]
- Lanfear R, Frandsen PB, Wright AM, et al. (2016). PartitionFinder 2: New methods for selecting Partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34: 772–773. [DOI] [PubMed] [Google Scholar]
- Larsson E, Vauras J, Cripps CL. (2014). Inocybe leiocephala, a species with an intercontinental distribution range: disentangling the I. leiocephala - subbrunnea - catalaunica morphological species complex. Karstenia 54: 15–39. [Google Scholar]
- Larsson E, Vauras J, Cripps CL. (2017). Inocybe lemmi, a new species of section Marginatae from the alpine region of Sweden. Karstenia 57: 1–9. [Google Scholar]
- Latha KPD, Manimohan P. (2017). Inocybe of Kerala. Ph.D. dissertation. Department of Botany, University of Calicut, Kerala, India. [Google Scholar]
- Latha KPD, Manimohan P, Matheny PB. (2016). A new species of Inocybe representing the Nothocybe lineage. Phytotaxa 267: 040–050. [Google Scholar]
- Matheny B, Liu YJ, Ammirati JF, et al. (2002). Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89: 688–698. [DOI] [PubMed] [Google Scholar]
- Matheny PB. (2005). Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Molecular Phylogenetics and Evolution 35: 1–20. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Aime MC, Bougher NL, et al. (2009). Out of the Palaeotropics? Historical biogeography and diversification of the cosmopolitan ectomycorrhizal mushroom family Inocybaceae. Journal of Biogeography 36: 577–592. [Google Scholar]
- Matheny PB, Aime MC, Henkel TW. (2003). New species of Inocybe from Dicymbe forests of Guyana. Mycological Research 107: 495–505. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Bougher N. (2017). Fungi of Australia: Inocybaceae. Australian Biological Resources Study, Canberra. Melbourne: CSIRO Publishing, Canberra & Melbourne. [Google Scholar]
- Matheny PB, Henkel TW, Séné O, et al. (2017). New species of Auritella (Inocybaceae) from Cameroon, with a worldwide key to the known species. IMA Fungus 8: 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny PB, Hobbs AM, Esteve-Raventós F. (2020). Genera of Inocybaceae: New skin for the old ceremony. Mycologia 112 (1): 83–120. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Kudzma LV. (2019). New species of Inocybe (Inocybaceae) from eastern North America1. The Journal of the Torrey Botanical Society 146: 213–235. [Google Scholar]
- Matheny PB, Moreau PA. (2009) A rare and unusual lignicolous species of Inocybe (Agaricales) from eastern North America. Brittonia 61: 163–171. [Google Scholar]
- Matheny PB, Norvell LL, Giles EC. (2013). A common new species of Inocybe in the Pacific Northwest with a diagnostic PDAB reaction. Mycologia 105: 436–446. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010) (November). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Gateway Computing Environments Workshop (GCE), 2010. New Orleans, LA: 1–8. [Google Scholar]
- Nguyen LT, Schmidt HA, von Haeseler A, et al. (2015). IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida F. (1989). Key to the species of Inocybe in California. Mycotaxon 34:181–196. [Google Scholar]
- Online Auction Color C (2004). The New Language of Color for Buyers and Sellers! www.OnlineAuctionColorChart.com. [Google Scholar]
- Osmundson TW, Robert VA, Schoch CL, et al. (2013). Filling Gaps in Biodiversity Knowledge for Macrofungi: Contributions and Assessment of an Herbarium Collection DNA Barcode Sequencing Project. PLoS ONE 8: e62419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouabbou A, Touhami AO, Benkirane R, et al. (2014). Study of some new species of the Inocybe genus with gibbous spore for the Morocco’s fungal flora. International Journal of Recent Scientific Research 5: 1464–1467. [Google Scholar]
- Pegler DN. (1969). Studies on African Agaricales: II. Kew Bulletin 23: 219–249. [Google Scholar]
- Rehner S, Samuels G. (1995). Molecular Systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorph. Canadian Journal of Botany 73: 816–823. [Google Scholar]
- Ronquist F, Teslenko M, Van Der Mark P, et al. (2012). MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg M, Larsson E, Jacobsson S. (2010). An evolutionary perspective on morphological and ecological characters in the mushroom family Inocybaceae (Agaricomycotina, Fungi). Molecular Phylogenetics and Evolution 55: 431–442. [DOI] [PubMed] [Google Scholar]
- Ryberg M, Matheny PB. (2012). Asynchronous origins of ectomycorrhizal clades of Agaricales. Proceedings of the Royal Society B: Biological Sciences 279: 2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg M, Nilsson RH, Kristiansson E, et al. (2008). Mining metadata from unidentified ITS sequences in GenBank: A case study in Inocybe (Basidiomycota). BMC Evolutionary Biology 8: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seress D, Dima B, Kovács GM. (2016). Characterisation of seven Inocybe ectomycorrhizal morphotypes from a semiarid woody steppe. Mycorrhiza 26: 215–225. [DOI] [PubMed] [Google Scholar]
- Singer R. (1986). The Agaricales in modern taxonomy, (4th Edn.). Koeltz Scientific Books, Koenigstein. [Google Scholar]
- Tedersoo L, Bahram M, Jairus T, et al. (2011). Spatial structure and the effects of host and soil environments on communities of ectomycorrhizal fungi in wooded savannas and rain forests of Continental Africa and Madagascar. Molecular Ecology 20: 3071–3080. [DOI] [PubMed] [Google Scholar]
- Vauras J, Larsson E. (2015). Inocybe caprimulgi and I. lacunarum, two new nodulose-spored species from Fennoscandia. Karstenia 55: 1–18. [Google Scholar]
- Vauras J, Larsson E. (2016). Inocybe baltica and I. suecica, two new smooth-spored species from the Baltic Sea region. Karstenia 56: 13–26. [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds. PCR protocols: a guide to methods and applications. New York: Academic Press: 315–322. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ML tree of ITS+28S sequences showing the placement of Inocybe beninensis, I. flavipes I. fuscobrunnea and I. pallidiangulata. For each node, the ML ultrafast bootstrap support ≥ 95 % is presented above or in front of the branch leading to that node.
ML tree of RPB2 sequences showing the placement of Inocybe flavipes, I. fuscobrunnea and I. pallidiangulata. For each node, the ML ultrafast bootstrap support ≥ 95 % is presented above or in front of the branch leading to that node.
ML tree showing the placement of four new species described from tropical regions of Africa: Inocybe beninensis, I. flavipes I. fuscobrunnea and I. pallidiangulata based on phylogenetic analyses of ITS, 28S and RPB2 data set.


