Abstract
Nine new genera, 17 new species, nine new combinations, seven epitypes, three lectotypes, one neotype, and 14 interesting new host and / or geographical records are introduced in this study. New genera: Neobarrmaelia (based on Neobarrmaelia hyphaenes), Neobryochiton (based on Neobryochiton narthecii), Neocamarographium (based on Neocamarographium carpini), Nothocladosporium (based on Nothocladosporium syzygii), Nothopseudocercospora (based on Nothopseudocercospora dictamni), Paracamarographium (based on Paracamarographium koreanum), Pseudohormonema (based on Pseudohormonema sordidus), Quasiphoma (based on Quasiphoma hyphaenes), Rapidomyces (based on Rapidomyces narthecii). New species: Ascocorticium sorbicola (on leaves of Sorbus aucuparia, Belgium), Dactylaria retrophylli (on leaves of Retrophyllum rospigliosii, Colombia), Dactylellina miltoniae (on twigs of Miltonia clowesii, Colombia), Exophiala eucalyptigena (on dead leaves of Eucalyptus viminalis subsp. viminalis supporting Idolothrips spectrum, Australia), Idriellomyces syzygii (on leaves of Syzygium chordatum, South Africa), Microcera lichenicola (on Parmelia sulcata, Netherlands), Neobarrmaelia hyphaenes (on leaves of Hyphaene sp., South Africa), Neobryochiton narthecii (on dead leaves of Narthecium ossifragum, Netherlands), Niesslia pseudoexilis (on dead leaf of Quercus petraea, Serbia), Nothocladosporium syzygii (on leaves of Syzygium chordatum, South Africa), Nothotrimmatostroma corymbiae (on leaves of Corymbia henryi, South Africa), Phaeosphaeria hyphaenes (on leaves of Hyphaene sp., South Africa), Pseudohormonema sordidus (on a from human pacemaker, USA), Quasiphoma hyphaenes (on leaves of Hyphaene sp., South Africa), Rapidomyces narthecii (on dead leaves of Narthecium ossifragum, Netherlands), Reticulascus parahennebertii (on dead culm of Juncus inflexus, Netherlands), Scytalidium philadelphianum (from compressed air in a factory, USA). New combinations: Neobarrmaelia serenoae, Nothopseudocercospora dictamni, Dothiora viticola, Floricola sulcata, Neocamarographium carpini, Paracamarographium koreanum, Rhexocercosporidium bellocense, Russula lilacina. Epitypes: Elsinoe corni (on leaves of Cornus florida, USA), Leptopeltis litigiosa (on dead leaf fronds of Pteridium aquilinum, Netherlands), Nothopseudocercospora dictamni (on living leaves of Dictamnus albus, Russia), Ramularia arvensis (on leaves of Potentilla reptans, Netherlands), Rhexocercosporidium bellocense (on leaves of Verbascum sp., Germany), Rhopographus filicinus (on dead leaf fronds of Pteridium aquilinum, Netherlands), Septoria robiniae (on leaves of Robinia pseudoacacia, Belgium). Lectotypes: Leptopeltis litigiosa (on Pteridium aquilinum, France), Rhopographus filicinus (on dead leaf fronds of Pteridium aquilinum, Netherlands), Septoria robiniae (on leaves of Robinia pseudoacacia, Belgium). Neotype: Camarographium stephensii (on dead leaf fronds of Pteridium aquilinum, Netherlands).
Citation: Crous PW, Begoude BAD, Boers J, Braun U, Declercq B, Dijksterhuis J, Elliott TF, Garay-Rodriguez GA, Jurjević Ž, Kruse J, Linde CC, Loyd A, Mound L, Osieck ER, Rivera-Vargas LI, Quimbita AM, Rodas CA, Roux J, Schumacher RK, Starink-Willemse M, Thangavel R, Trappe JM, van Iperen AL, Van Steenwinkel C, Wells A, Wingfield MJ, Yilmaz N, Groenewald JZ (2022) New and Interesting Fungi. 5. Fungal Systematics and Evolution 10: 19–90. doi: 10.3114/fuse.2022.10.02
Keywords: biodiversity, ITS barcodes, multi-gene phylogeny, new taxa, systematics, typification
INTRODUCTION
The present study represents the fifth instalment of the New and Interesting Fungi (NIF) series that is published annually in the journal Fungal Systematics and Evolution. Papers report new knowledge on fungal biodiversity, list new host or geographical records, and new sexual-asexual connections. This study also includes validations and descriptions of new fungal taxa and lists interesting observations relating to fungal biology. Mycologists and other researchers wishing to contribute to future issues of NIF are encouraged to contact the Editor-in-Chief (p.crous@wi.knaw.nl).
MATERIALS AND METHODS
Isolates
Twig, culm and leaf samples (see Table 1) were treated as previously detailed (Crous et al. 2019b). Single conidial colonies were established on Petri dishes containing 2 % malt extract agar (MEA) as described by Crous et al. (1991), and single ascospore cultures were established following the method described by Crous (1998). Colonies were sub-cultured on 2 % potato-dextrose agar (PDA), oatmeal agar (OA), MEA (Crous et al. 2019b), or autoclaved pine needles on 2 % tap water agar (PNA) (Smith et al. 1996), and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation. Reference strains and specimens of the studied fungi are maintained in the culture collection and fungarium (CBS) of the Westerdijk Fungal Biodiversity Institute (WI), Utrecht, the Netherlands.
Table 1.
Collection details and GenBank accession numbers of isolates treated in this study, and associated ex-type strains where available. Species for which additional sequences were generated during the course of this study are also listed here. Novel GenBank accession numbers are indicated in bold font.
| Species | Culture or voucher accession number(s)1 | Locality | Substrate | Collector(s) |
GenBank accession number2
|
||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | rpb2 | tub2 | Other loci | |||||
| Annellophorella ellisii | CBS 738.70 = IMI 140024, ex-type | France | Wood scrap on forest soil | – | ON811482.1 | MH871721.1 | – | – | – |
| Ascochyta nigripycnidia | CBS 116.96 = CCMF 243 = PD 95/7930, ex-type | Czech Republic | Vicia cracca, leaf spot | M. Ondrej | NR_135978.1 | NG_070603.1 | MT018253.1 | GU237637.1 | – |
| CPC 39716 = CBS 148249 | Russia | Vicia tenuifolia | T.S. Bulgakov | ON811483.1 | ON811542.1 | ON803534.1 | – | tef1 (first part): ON803560.1 | |
| Ascocorticium sorbicola, gen. et sp. nov. | CPC 40075 = CBS 148303, ex-type | Belgium | Sorbus aucuparia, leaves | B. Declercq | ON811506.1 | ON811565.1 | ON803545.1 | – | tef1 (second part): ON803584.1 |
| Camarographium stephensii | CPC 41598 = CBS 149168, ex-neotype | Netherlands | Pteridium aquilinum, dead leaf fronds | P.W. Crous | ON811484.1 | ON811543.1 | – | ON803587.1 | tef1 (first part): ON803561.1 |
| CPC 41923 | Netherlands | Pteridium aquilinum, stem | P.W. Crous | ON811485.1 | ON811544.1 | – | ON803588.1 | tef1 (first part): ON803562.1 | |
| Cercosporidium chaetomium | CPC 18624 = CBS 142177, ex-epitype | Canada | Euphorbia sp. | P.W. Crous & K. Seifert | NR_156367.1 | NG_069527.1 | MF951474.1 | – | – |
| CPC 39688 = CBS 148457 | Russia | Polygonum aviculare | T.S. Bulgakov | ON811486.1 | ON811545.1 | ON803535.1 | – | – | |
| Coniella eucalyptorum | CPC 3904 = CBS 112640 = DFR 100185, ex-type | Australia | Eucalyptus grandis x tereticornis | P.Q. Thu & R.J. Gibbs | AY339338.1 | AY339290.1 | KX833452.1 | – | tef1 (first part): KX833637.1 |
| CPC 39780 = CBS 149182 | South Africa | Eucalyptus benthamii, leaves | J. Roux | ON811487.1 | ON811546.1 | – | ON803589.1 | tef1 (first part): ON803563.1 | |
| Curvularia eragrostidicola | BRIP 12538, ex-type | Australia | Eragrostis pilosa, inflorescence | J.L. Alcorn | NR_158446.1 | – | – | – | gapdh: MH433643.1, tef1 (first part): MH433661.1 |
| CPC 39009 = CBS 147069 | Namibia | Dung of Procavia sp. | P.W. Crous | ON811488.1 | ON811547.1 | ON803536.1 | – | tef1 (second part): ON803581.1 | |
| Dactylaria retrophylli, sp. nov | CPC 39510 = CBS 148271, ex-type | Colombia | Retrophyllum rospigliosii | M.J. Wingfield | ON811489.1 | ON811548.1 | – | – | – |
| Dactylellina miltoniae, sp. nov. | CPC 39508 = CBS 148270, ex-type | Colombia | Miltonia clowesii, twigs | M.J. Wingfield | ON811490.1 | ON811549.1 | ON803537.1 | – | tef1 (second part): ON803582.1 |
| Dothiora viticola, comb. nov. | CBS 140676 = FMR 13040 = L9D-17, ex-type | Spain: Canary Islands | Vitis vinifera cv. Malvasia, fruit (grapes) | F. Laich | NR_137620.1 | MH878164.1 | – | – | – |
| Elsinoe corni | CPC 41728 = CBS 148184, ex-epitype | USA: North Carolina | Cornus florida | A. Loyd | ON811491.1 | ON811550.1 | ON803538.1 | – | – |
| Elsinoe parthenocissi | CPC 38770 = T19_06384B = CBS 146969 | New Zealand | Parthenocissus quinquefolia | C. Inglis | ON811492.1 | ON811551.1 | ON803539.1 | – | – |
| Exophiala eucalyptigena, sp. nov. | CPC 41024 = CBS 148273, ex-type | Australia | Dead leaves of Eucalyptus viminalis subsp. viminalis supporting Idolothrips spectrum population | A. Wells & L.A. Mound | ON811493.1 | ON811552.1 | – | ON803590.1 | tef1 (first part): ON803564.1 |
| Floricola juncicola | CPC 38197 = CBS 146811, ex-type | France | Juncus sp., dead culm | A. Gardiennet | NR_173010.1 | NG_076710.1 | MW890063.1 | – | tef1 (first / second part): MW890092.1 / MW890108.1 |
| CPC 41356 = CBS 148318 | Netherlands | Juncus effusus, dead culm | E.R. Osieck | ON811494.1 | ON811553.1 | ON803540.1 | – | tef1 (first part): ON803565.1 | |
| CPC 41357 = CBS 148287 | Netherlands | Juncus effusus, dead culm | E.R. Osieck | ON811495.1 | ON811554.1 | ON803541.1 | – | tef1 (first part): ON803566.1 | |
| Floricola sulcata, comb. nov. | CBS 118224 = CMW 18063, ex-type | South Africa | Ischyrolepis subverticellata, dead culm | S. Marincowitz | JX517284.1 | JX517293.1 | – | – | – |
| CPC 41345 = CBS 148286 | Netherlands | Juncus effusus, dead culm | E.R. Osieck | ON811496.1 | ON811555.1 | ON803542.1 | – | tef1 (first part): ON803567.1 | |
| Idriellomyces syzygii, sp. nov. | CPC 40065 = CBS 148252, ex-type | South Africa | Syzygium cordatum, leaves | M.J. Wingfield | ON811497.1 | ON811556.1 | – | – | – |
| Leptopeltis litigiosa | CPC 41927 = CBS 149171, ex-epitype | Netherlands | Pteridium aquilinum, dead leaf fronds | P.W. Crous | ON811498.1 | ON811557.1 | – | – | – |
| Macgarvieomyces juncicola | CPC 40815 = CBS 148264 | Netherlands | Juncus effusus, dead culm | E.R. Osieck | ON811499.1 | ON811558.1 | – | – | actA: ON803512.1, cmdA: ON803526.1, rpb1: ON803532.1 |
| Magnibotryascoma mali | CPC 38756 = T19_05741A = CBS 147001 | New Zealand | Metrosideros sp. | L. Rabbidge | ON811500.1 | ON811559.1 | – | – | tef1 (first part): ON803568.1 |
| CPC 38757 = T19_05741B = CBS 146778 | New Zealand | Metrosideros sp. | L. Rabbidge | ON811501.1 | ON811560.1 | – | – | tef1 (first / second part): ON803569.1 / ON803583.1 | |
| MFLUCC 17-0933, ex-type | China | Malus halliana, decaying twigs | C. Phukhamsakda | NR_156346.1 | NG_059830.1 | MF173437.1 | – | SSU: NG_063644.1, tef1 (second part): MF173435.1 | |
| Microcera lichenicola, sp. nov. | CPC 41114 = CBS 149169, ex-type | Netherlands | Parmelia sulcata | J. Boers | ON811502.1 | ON811561.1 | – | ON803591.1 | – |
| Microcera sp. | CPC 41230 = CBS 148313 | Netherlands | Physcia tenella | J. Boers | ON811503.1 | ON811562.1 | ON803543.1 | ON803592.1 | rpb1: ON803533.1, tef1 (first part): ON803570.1 |
| Mycodiella eucalypti | CPC 29458 = CBS 142098, ex-type | Australia | Eucalyptus diversicolor, leaves | P.W. Crous | NR_155408.1 | NG_059747.1 | KY173586.1 | – | actA: KY173565.1 |
| CPC 38962 = CBS 146986 | South Africa | Syzygium cordatum, leaf litter | P.W. Crous | ON811505.1 | ON811564.1 | ON803544.1 | ON803593.1 | cmdA: ON803527.1 | |
| Neobarrmaelia hyphaenes, gen. et sp. nov. | CPC 40101 = CBS 148304, ex-type | South Africa | Hyphaene crenata, leaves | M.J. Wingfield & J. Roux | ON811507.1 | ON811566.1 | ON803546.1 | ON803594.1 | – |
| Neobarrmaelia serenoae, comb. nov. | CPC 37572 = CBS 146017, ex-type | USA | Serenoa repens, leaf | M.J. Wingfield | MT223781.1 | MT223876.1 | – | MT223730.1 | tef1 (first part): MT223709.1 |
| Neobryochiton narthecii, gen. et sp. nov. | CPC 41972 = CBS 149172, ex-type | Netherlands | Narthecium ossifragum, dead leaves | J. Boers | ON811508.1 | ON811567.1 | ON803547.1 | – | – |
| Neocamarographium carpini, gen. et comb. nov. | CPC 18919, 18918 = CBS 128781, ex-isotype | Russia | Carpinus betulus, thin, dried twigs | V. Mel’nik | NR_156250.1 | NG_058837.1 | – | – | – |
| Neocamarographium carpini, gen. et comb. nov. | CPC 25067 | Germany | Carpinus betulus,attached twig | R.K. Schumacher | ON811509.1 | – | – | – | – |
| Neofusicoccum mediterraneum | CMW 26679 = CBS 125263 | South Africa | Terminalia sericea | D. Begoude & J. Roux | MH863478.1 | MH874970.1 | KX464045.1 | KX465052.1 | tef1 (first part): GQ471780.1 |
| CPC 13137 = CBS 121718, ex-type | Greece | Eucalyptus sp. branches and leaves | P.W. Crous, M.J. Wingfield & A.J.L. Phillips | EU040221.1 | EU040221.1 | KY855815.1 | – | actA: KY855639.1, gapdh: KY855694.1 | |
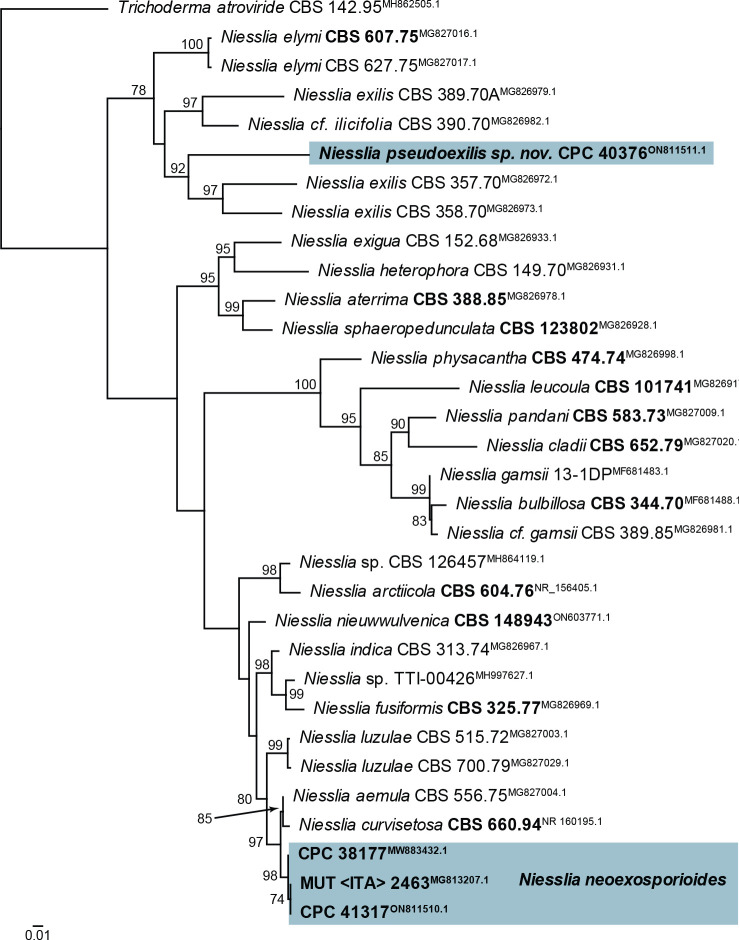
| Niesslia neoexosporioides | CPC 38177 = CBS 146810, ex-type | Germany | Carex paniculata, dead leaves | R.K. Schumacher | NR_173014.1 | NG_076714.1 | – | MW890137.1 | actA: MW890027.1, tef1 (first part): MW890097.1 |
| CPC 41317 = CBS 148284 | Netherlands | Phragmites australis, dead culms | E.R. Osieck | ON811510.1 | ON811568.1 | – | ON803595.1 | actA: ON803513.1, tef1 (first part): ON803571.1 | |
| Niesslia pseudoexilis, sp. nov. | CPC 40376 = CBS 148333, ex-type | Serbia | Quercus petraea, dead leaf | D. Savić | ON811511.1 | ON811569.1 | ON803548.1 | ON803596.1 | actA: ON803514.1 |
| Nothocladosporium syzygii, gen. et sp. nov. | CPC 40091 = CBS 148289, ex-type | South Africa | Syzygium cordatum, leaves | M.J. Wingfield | ON811512.1 | ON811570.1 | – | ON803597.1 | actA: ON803515.1, tef1 (first part): ON803572.1 |
| Nothopseudocercospora dictamni, gen. et comb. nov. | CPC 39776 = CBS 148299, ex-epitype | Russia | Dictamnus albus | T.S. Bulgakov | ON811513.1 | ON811571.1 | ON803549.1 | – | actA: ON803516.1, cmdA: ON803528.1, tef1 (first part): ON803573.1 |
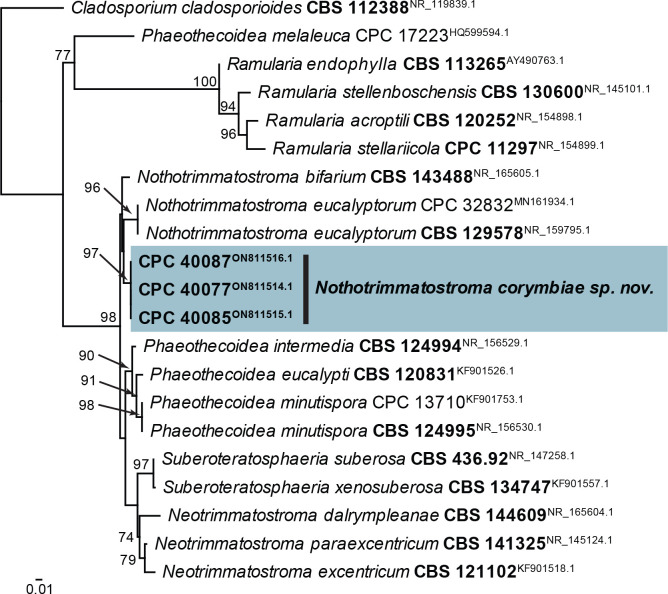
| Nothotrimmatostroma corymbiae, sp. nov. | CPC 40077 = CBS 148336 | South Africa | Corymbia henryi | M.J. Wingfield | ON811514.1 | ON811572.1 | – | – | – |
| CPC 40085 = CBS 148335 | South Africa | Corymbia henryi | M.J. Wingfield | ON811515.1 | ON811573.1 | – | – | – | |
| CPC 40087 = CBS 148334, ex-type | South Africa | Corymbia henryi | M.J. Wingfield & J. Roux | ON811516.1 | ON811574.1 | – | – | – | |
| Paracamarographium koreanum, gen. et comb. nov. | CBS 117159, ex-type | Korea | Cornus kousa, dead twigs | V. Mel’nik | JQ044432.1 | JQ044451.1 | – | – | – |
| Paraphoma salicis | CPC 38651 = CBS 146797 = CWU AS 7121, ex-type | Ukraine | Salix cf. alba, leaves | A. Akulov | NR_173017.1 | MW883829.1 | MW890069.1 | MW890140.1 | actA: MW890028.1 |
| CPC 39991 = CBS 148454 | Netherlands | Salix sp. | A.L. van Iperen | ON811517.1 | ON811575.1 | ON803550.1 | ON803598.1 | – | |
| Periconia pseudobyssoides | BILAS 50334 = S1-11P, ex-type | Lithuania | Heracleum sosnowskyi, dead stalks | S. Markovskaja | KC954161.1 | – | – | – | – |
| CPC 38820 = CBS 147067 | South Africa | Euphorbia ingens, leaf litter | P.W. Crous | ON811518.1 | ON811576.1 | – | – | tef1 (first / second part): ON803574.1 / ON803585.1 | |
| Phaeoisaria clematidis | CPC 39680 | Netherlands | Sambucus nigra, stems | P.W. Crous | ON811519.1 | ON811577.1 | ON803551.1 | – | SSU: ON787969.1 |
| CPC 39937 = CBS 149173 | Netherlands | Sambucus nigra, stems | A.L. van Iperen | ON811520.1 | ON811578.1 | ON803552.1 | – | – | |
| Phaeophleospora hymenocallidicola | CPC 25014 = CBS 139912, ex-type | Thailand | Leaves of epiphyte | P.W. Crous | NR_137994.1 | NG_070062.1 | – | – | – |
| CPC 40026 = CBS 148250 | USA: Puerto Rico | Mangifera indica var. palmer, leaf necrosis | – | ON811521.1 | ON811579.1 | – | – | – | |
| CPC 40027 = CBS 149175 | USA: Puerto Rico | Necrosis on fruit peduncle of Mangifera indica | – | ON811522.1 | – | – | – | – | |
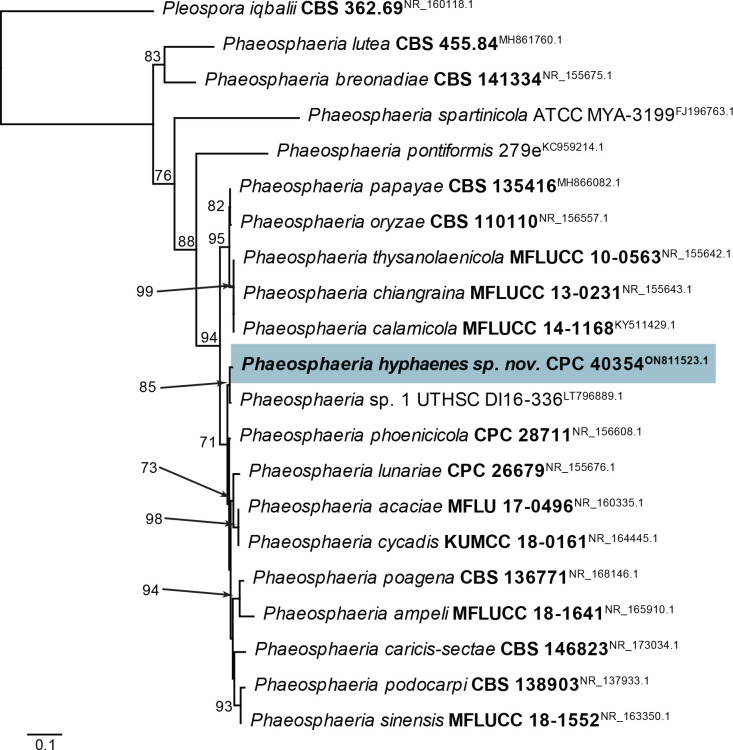
| Phaeosphaeria hyphaenes, sp. nov. | CPC 40354 = CBS 148254, ex-type | South Africa | Hyphaene crenata, leaves | M.J. Wingfield & J. Roux | ON811523.1 | ON811580.1 | ON803553.1 | ON803599.1 | tef1 (first part): ON803575.1 |
| Phlyctema phoenicis | CPC 29372 = T15_05353B = CBS 142134, ex-type | New Zealand | Phoenix canariensis | R. Thangaval | NR_155690.1 | NG_067319.1 | – | KY173611.1 | – |
| CPC 38747 = CBS 147066 | New Zealand | Libertia ixioides | C. Inglis | ON811524.1 | ON811581.1 | – | ON803600.1 | actA: ON803517.1 | |
| Pseudohormonema sordidus, gen. et sp. nov. | CBS 130468 = UTHSC 07-2004, ex-type | USA | Human pacemaker | D. Sutton | ON811525.1 | ON811582.1 | – | ON803601.1 | actA: ON803518.1 |
| CBS 140365 | Saudi Arabia | Polluted soil | S. de Hoog & T.A. Moussa | ON811526.1 | ON811583.1 | – | ON803602.1 | – | |
| CBS 140366 | Saudi Arabia | Polluted soil | S. de Hoog & T.A. Moussa | ON811527.1 | – | – | ON803603.1 | – | |
| Pseudoplagiostoma eucalypti | CPC 13341 = CBS 124807, ex-type | Venezuela | Eucalyptus urophylla, living leaves | M.J. Wingfield | GU973512.1 | GU973606.1 | – | GU973575.1 | tef1 (first part): GU973542.1 |
| CPC 39762 = CBS 149183 | South Africa | Eucalyptus benthamii, leaves | J. Roux | ON811528.1 | ON811584.1 | – | ON803604.1 | tef1 (first part): ON803576.1 | |
| Pseudosoloacrosporiella cryptomeriae | CPC 39587 = CBS 148441, ex-type | Netherlands | Cryptomeria japonica, leaves | P.W. Crous | NR_175206.1 | ON811585.1 | – | – | tef1 (first part): OK651183.1 |
| Pseudosydowia phantasmae, comb. nov. | CPC 38883 = CBS 146830, ex-type | Namibia | Moringa ovalifolia, leaves | P.W. Crous | NR_171999.1 | NG_074499.1 | – | – | – |
| CPC 38950 = CBS 146982 | Namibia | Moringa ovalifolia, flower | P.W. Crous | ON811504.1 | ON811563.1 | – | – | – | |
| Quasiphoma hyphaenes, gen. et sp. nov. | CPC 40045 = CBS 148253, ex-type | South Africa | Hyphaene crenata, leaves | M.J. Wingfield & J. Roux | ON811529.1 | ON811586.1 | ON803554.1 | ON803605.1 | actA: ON803519.1 |
| Racheliella wingfieldiana | CPC 13806 = CBS 143669, ex-type | South Africa | Syzygium guineense | M.J. Wingfield | MG591911.1 | MG592006.1 | MG976487.1 | MG592192.1 | tef1 (first part): MG592100.1 |
| CPC 40039 = CBS 148251 | South Africa | Syzygium sp. | M.J. Wingfield | ON811530.1 | ON811587.1 | – | ON803606.1 | tef1 (first part): ON803577.1 | |
| Ramularia arvensis | CPC 39985 = CBS 148455, ex-epitype | Netherlands | Potentilla reptans | A.L. van Iperen | ON811531.1 | ON811588.1 | – | – | actA: ON803520.1, gapdh: ON803530.1, his3: ON803531.1, tef1 (first part): ON803578.1 |
| Rapidomyces narthecii, gen. et sp. nov. | CPC 41974 = CBS 149174, ex-type | Netherlands | Narthecium ossifragum, dead leaves | J. Boers | ON811532.1 | ON811589.1 | ON803555.1 | – | – |
| Reticulascus parahennebertii, sp. nov. | CPC 41226 = CBS 148282, ex-type | Netherlands | Juncus inflexus, dead culm | E.R. Osieck | ON811533.1 | ON811590.1 | – | – | tef1 (second part): ON803586.1 |
| Rhexocercosporidium bellocense, comb. nov. | CPC 39764 = CBS 148297, ex-epitype | Germany | Verbascum cf. densiflorum | J. Kruse | ON811535.1 | ON811592.1 | ON803557.1 | – | – |
| Rhexocercosporidium aff. bellocense | CPC 39690 = CBS 148246 | Russia | Verbascum sp. | T.S. Bulgakov | ON811534.1 | ON811591.1 | ON803556.1 | ON803607.1 | – |
| Rhopographus filicinus | CBS 384.59 = ETH 2752 | India | Pteridium aquilinum | E. Müller | MH857898.1 | ON811593.1 | – | – | – |
| CPC 41596, ex-epitype | Netherlands | Pteridium aquilinum, dead leaf fronds | P.W. Crous | ON811536.1 | ON811594.1 | ON803558.1 | – | actA: ON803521.1 | |
| CPC 41925 | Netherlands | Pteridium aquilinum, stem | P.W. Crous | ON811537.1 | ON811595.1 | – | – | actA: ON803522.1 | |
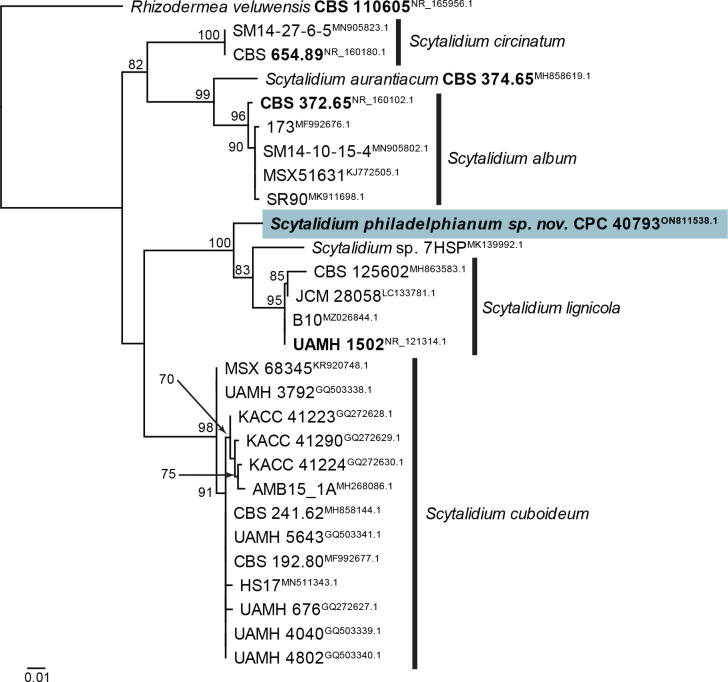
| Scytalidium philadelphianum, sp. nov. | CPC 40793 = CBS 148262, ex-type | USA: Pennsylvania | Compressed air in a factory | Ž. Jurjević | ON811538.1 | – | – | – | – |
| Septoria chelidonii | CPC 12337 = CBS 132027 | South Korea | Chelidonium majus | H.D. Shin | GU269860.1 | GU253870.1 | KF252374.1 | KF252845.1 | actA: KF253676.1, cmdA: KF254025.1, tef1 (first part): KF253319.1 |
| CPC 39746 = CBS 148456 | Russia | Chelidonium majus | T.S. Bulgakov | ON811539.1 | ON811596.1 | – | – | actA: ON803523.1, tef1 (first part): ON803579.1 | |
| Septoria robiniae | CPC 39783 = CBS 148300, ex-epitype | Belgium | Robinia pseudoacacia | C. van Steenwinkel | ON811540.1 | ON811597.1 | – | – | actA: ON803524.1, tef1 (first part): ON803580.1 |
| Teratosphaeria alcornii | CPC 13384 = CBS 121100, ex-epitype | Australia | Corymbia variegata, leaves | G. Price | EF394866.1 | KF901882.1 | KF902407.1 | KF902982.1 | actA: KF903646.1, cmdA: KF902698.1 |
| CPC 39789 = CBS 149184 | South Africa | Corymbia citriodora, leaves | J. Roux | ON811541.1 | ON811598.1 | ON803559.1 | ON803608.1 | actA: ON803525.1, cmdA: ON803529.1 | |
1BILAS: Herbarium of Institute of Botany, Nature Research Centre, Vilnius, Lithuania; BRIP: Plant Pathology Herbarium, Department of Primary Industries, Queensland, Australia; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CMW: Culture Collection of the Forestry and Agricultural Biotechnology Institute (FABI) of the University of Pretoria, Pretoria, South Africa; CPC: Culture collection of Pedro Crous, housed at CBS; ETH: Swiss Federal Institute of Technology Culture Collection, Zurich, Switzerland; FMR: Facultat de Medicina, Universitat Rovira i Virgili, Reus, Spain; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; MUCL: Université Catholique de Louvain, Louvain-la-Neuve, Belgium; UTHSC: Fungus Testing Laboratory at the University of Texas Health Science Center, San Antonio, TX, USA.
2ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: large subunit (28S) of the nrRNA gene operon; act: partial actin gene; cmdA: partial calmodulin gene; gapdh: partial glyceraldehyde-3-phosphate dehydrogenase gene; his3: partial histone H3 gene; rpb1: partial DNA-directed RNA polymerase II largest subunit gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; SSU: small subunit (18S) of the nrRNA gene operon; tef1: partial translation elongation factor 1-alpha gene; tub2: partial beta-tubulin gene.
DNA extraction, amplification (PCR) and phylogeny
Fungal mycelium (Table 1) was scraped from the surface of agar cultures with a sterile scalpel and the genomic DNA was isolated using the Wizard® Genomic DNA Purification Kit (Promega Corporation, WI, USA) following the manufacturers’ protocols. All loci were amplified following previously published protocols. First, the 28S nrRNA gene (LSU) and internal transcribed spacer regions with intervening 5.8S nrRNA gene (ITS) of the nrDNA operon were sequenced for all the isolates included in this study (for amplification conditions, see Fan et al. 2018). Other loci were sequenced for various species or genera using primers and conditions specific for those groups of fungi. Amplification of the partial DNA-directed RNA polymerase II second largest subunit gene (rpb2), the partial translation elongation factor 1-alpha gene (tef1, first part) and the partial beta-tubulin gene (tub2) followed Braun et al. (2018), while amplification of the partial actin gene (actA), the partial calmodulin gene (cmdA), the partial glyceraldehyde-3-phosphate dehydrogenase gene (gapdh) and the partial histone H3 gene (his3) followed Videira et al. (2016). Amplification of the partial DNA-directed RNA polymerase II largest subunit gene (rpb1) followed Klaubauf et al. (2014), and the partial translation elongation factor 1-alpha gene (tef1, second part) followed Réblová et al. (2020). The resulting fragments were sequenced in both directions using the respective PCR primers and the BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems Life Technologies, Carlsbad, CA, USA); DNA sequencing amplicons were purified through Sephadex G-50 Superfine columns (Sigma-Aldrich, St. Louis, MO) in MultiScreen HV plates (Millipore, Billerica, MA). Purified sequence reactions were analysed on an Applied Biosystems 3730xl DNA Analyzer (Life Technologies, Carlsbad, CA, USA). The DNA sequences were analysed and consensus sequences were computed using Geneious Prime v. 2022.0.2 (http://www.geneious.com, Kearse et al. 2012).
The sequences for each gene region were subjected to megablast searches (Zhang et al. 2000) to identify closely related sequences in the NCBI’s GenBank nucleotide database. The results are provided as part of the species notes or as selected phylogenetic trees. Maximum-likelihood phylogenetic trees were constructed generated using IQ-TREE v. 2.1.3 (Nguyen et al. 2015, Minh et al. 2020) and branch support values were calculated with 5 000 ultrafast bootstrap replicates (Hoang et al. 2018) and optimal modelfinding using the TESTNEW option using ModelFinder (Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. The only exception was the Leotiomycetes LSU alignment which was analysed with MrBayes v. 3.2.7a (Ronquist et al. 2012) as explained in Braun et al. (2018) due to issues with longer branches in the IQ-TREE analysis. All resulting trees were printed with Geneious Prime v. 2022.0.2 and the layout of the trees was done in Adobe Illustrator 2022 v. 26.3.1.
Morphology
Slide preparations were mounted in lactic acid, Shear’s mounting fluid or water, from colonies sporulating on MEA, PDA, PNA or OA. Observations were made with a Nikon SMZ25 dissection-microscope, and with a Zeiss Axio Imager 2 light microscope using differential interference contrast (DIC) illumination and images recorded on a Nikon DS-Ri2 camera with associated software. Cryo Scanning Electron Microscopy methods followed Bensch et al. (2018). Colony characters and pigment production were noted after 2–4 wk of growth on MEA, PDA and OA (Crous et al. 2019b) incubated at 25 °C. Colony colours (surface and reverse) were scored using the colour charts of Rayner (1970). Sequences derived in this study were deposited in GenBank (Table 1), the alignments in figshare.com (doi: 10.6084/m9.figshare.20024564), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004).
RESULTS
Phylogeny
Statistics associated with the phylogenetic analyses presented in this study are provided in supplementary Table S1.
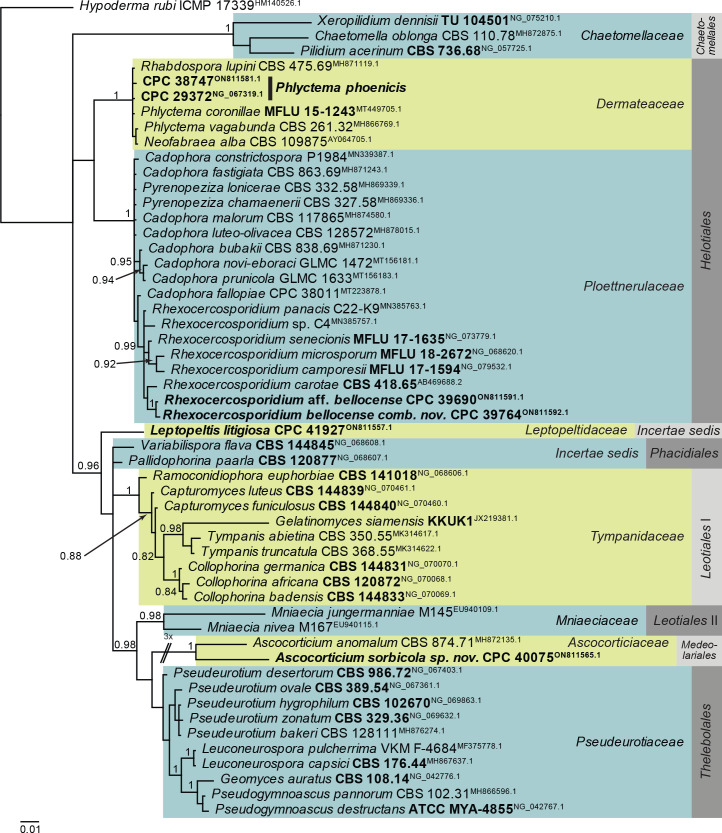
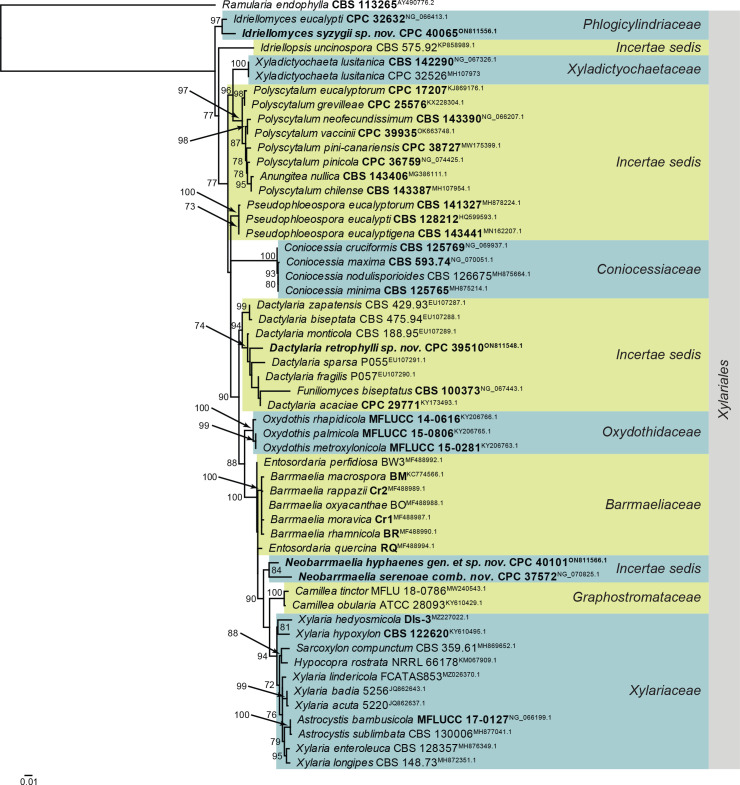
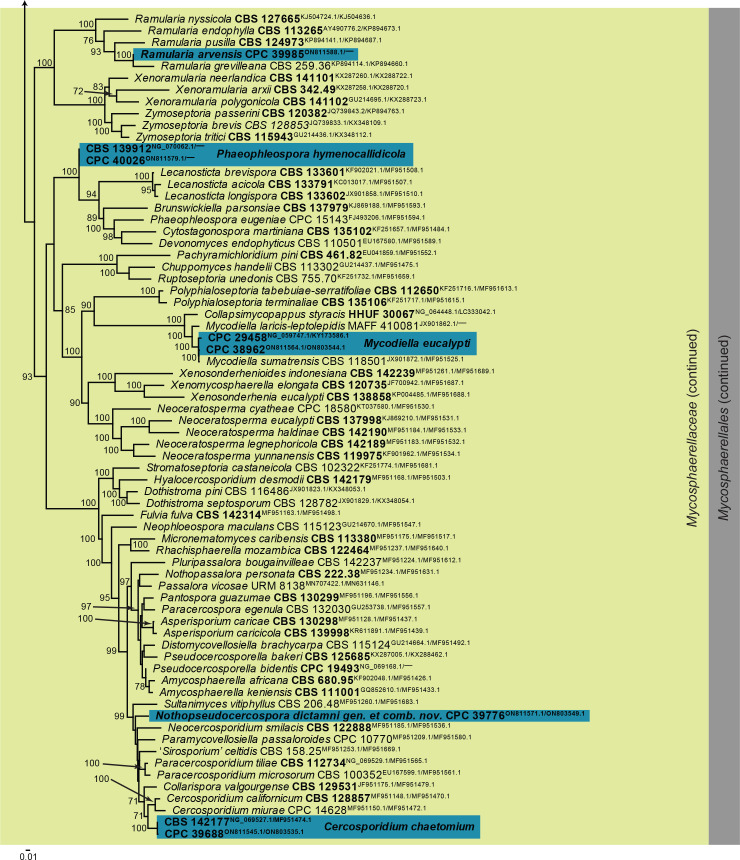
Overview phylogenies: Phylogenies based on LSU sequence alignments per class are provided for the species treated here and are discussed in the species notes where applicable. Some classes were split into order-specific trees to facilitate layout or improve the alignment. Overview phylogenies are provided for Dothideomycetes (diverse orders; Fig. 1, two parts), Dothideomycetes (Pleosporales; Fig. 2), Eurotiomycetes (Chaetothyriales; Fig. 3), Leotiomycetes (diverse orders; Fig. 4), Orbiliomycetes (Orbiliales; Fig. 5), Sordariomycetes (diverse orders; Fig. 6), Sordariomycetes (Hypocreales and Glomerales; Fig. 7), and Sordariomycetes (Xylariales; Fig. 8). The major differences between the IQ-TREE and MrBayes analyses of the Leotiomycetes LSU alignment pertained to the placement of, for example, Ascocorticiaceae (basal to Pseudeurotiaceae in the Bayesian analysis but terminal inside this family in the IQ-TREE analysis). Also, the positions of Chaetomellaceae and Phacidiales were not consistent between the two analyses, as is evident from the basal polytomies involving those lineages in Fig. 4.
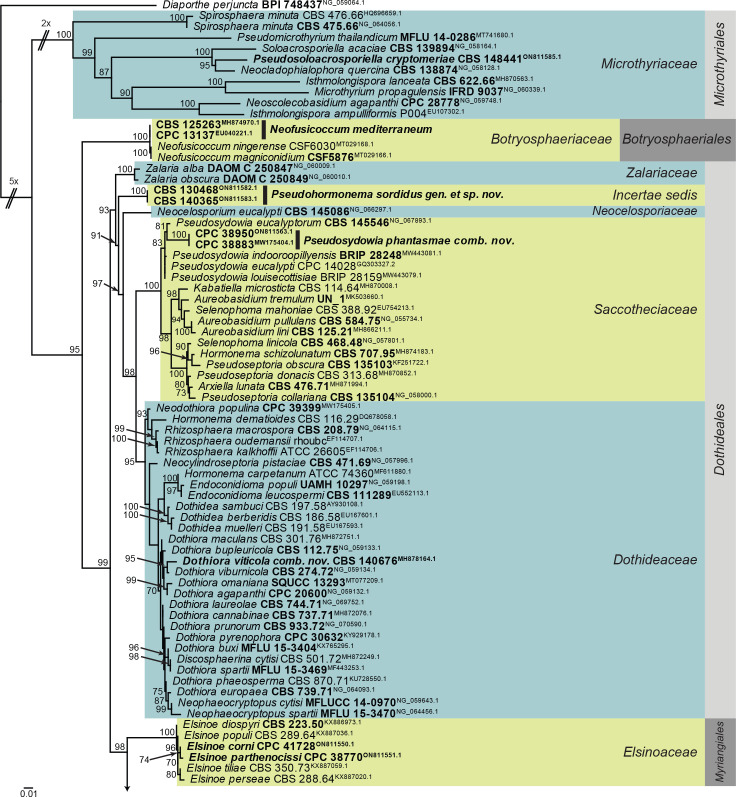
Fig. 1.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis of the Dothideomycetes (diverse orders) LSU nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Diaporthe perjuncta (voucher BPI 748437; GenBank NG_059064.1) and the species treated here are highlighted with coloured blocks and bold face. Accession numbers of sequence from material with a type status are also shown in bold face. Families and orders are indicated with coloured blocks to the right of the tree.
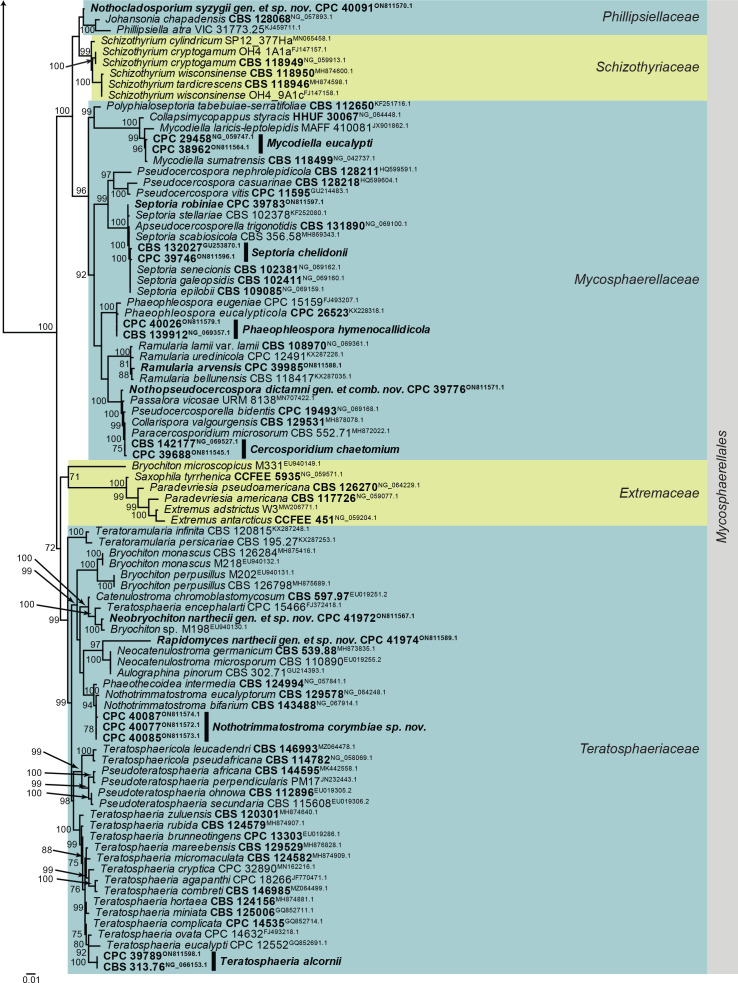
Fig. 2.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis of the Dothideomycetes (Pleosporales) LSU nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Xylaria hypoxylon (voucher OSC 100004; GenBank AY544648.1) and the species treated here are highlighted with coloured blocks and bold face. Accession numbers of sequence from material with a type status are also shown in bold face. Families and orders are indicated with coloured blocks to the right of the tree.
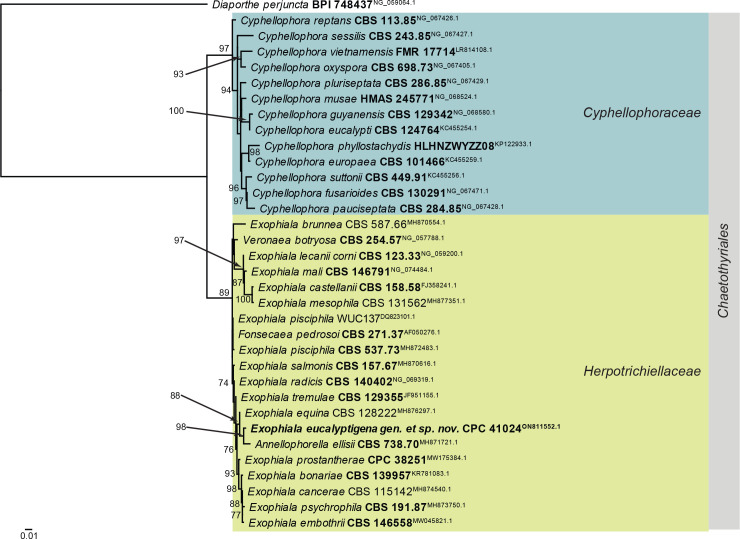
Fig. 3.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis of the Eurotiomycetes LSU nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Diaporthe perjuncta (voucher BPI 748437; GenBank NG_059064.1) and the species treated here is highlighted with bold face. Accession numbers of sequence from material with a type status are also shown in bold face. Families and orders are indicated with coloured blocks to the right of the tree.
Fig. 4.
Consensus phylogram (50 % majority rule) obtained from the Bayesian analysis of the Leotiomycetes LSU nucleotide alignment. Bayesian Posterior Probability values (> 0.79) are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Hypoderma rubi (culture ICMP 17339; GenBank HM140526.1) and the species treated here are highlighted with bold face. Accession numbers of sequence from material with a type status are also shown in bold face. Families and orders are indicated with coloured blocks to the right of the tree.
Fig. 5.
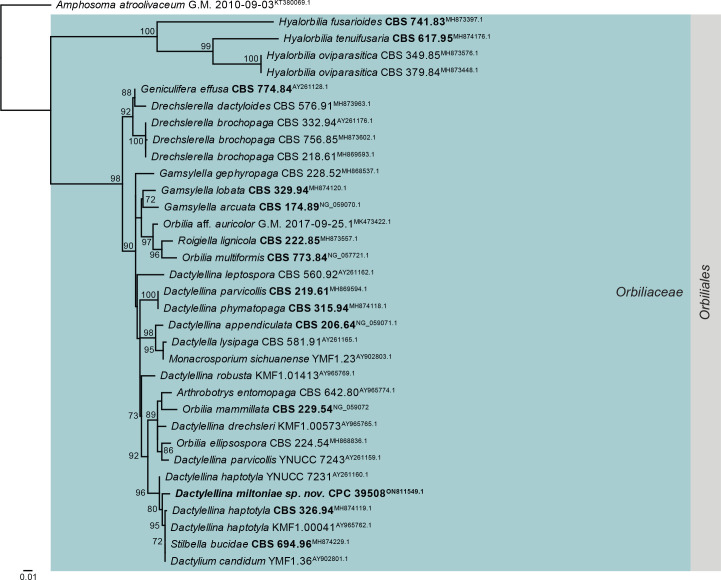
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 (Minh et al. 2020) of the Orbiliomycetes LSU nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Amphosoma atroolivaceum (voucher G.M. 2010-09-03; GenBank KT380069.1) and the species treated here is highlighted with bold face. Accession numbers of sequence from material with a type status are also shown in bold face. Families and orders are indicated with coloured blocks to the right of the tree.
Fig. 6.
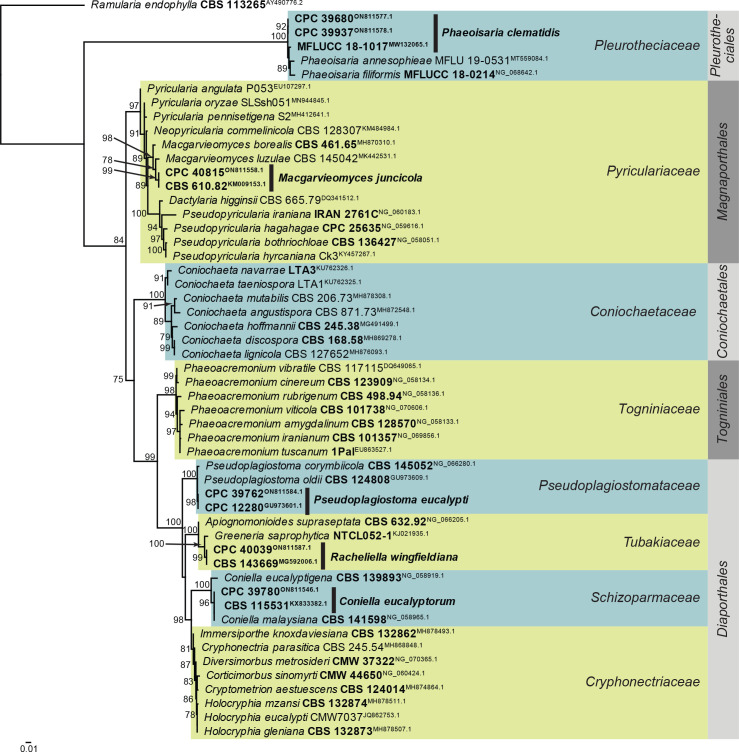
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis of the Sordariomycetes (diverse orders) LSU nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Ramularia endophylla (culture CBS 113265; GenBank AY490776.2) and the species treated here are highlighted with coloured blocks and bold face. Accession numbers of sequence from material with a type status are also shown in bold face. Families and orders are indicated with coloured blocks to the right of the tree.
Fig. 7.
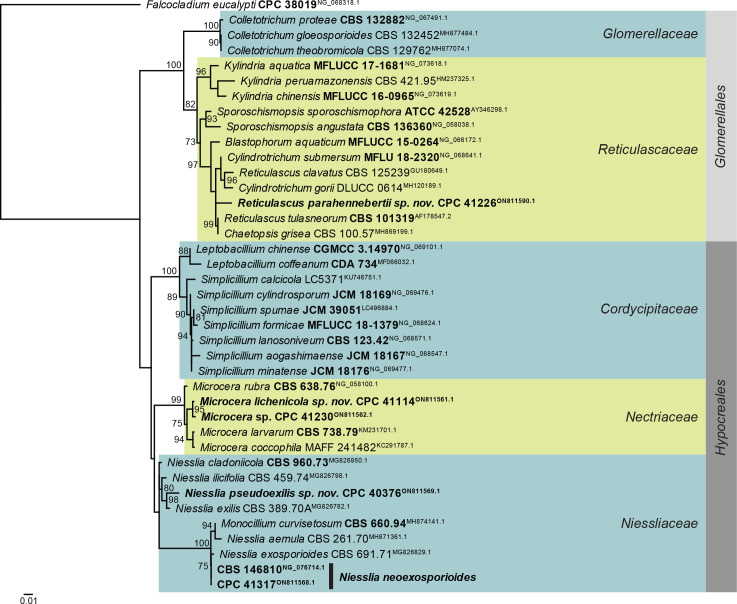
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis of the Sordariomycetes (Glomerales and Hypocreales) LSU nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Falcocladium eucalypti (culture CPC 38019; GenBank NG_068318.1) and the species treated here are highlighted with coloured blocks and bold face. Accession numbers of sequence from material with a type status are also shown in bold face. Families and orders are indicated with coloured blocks to the right of the tree.
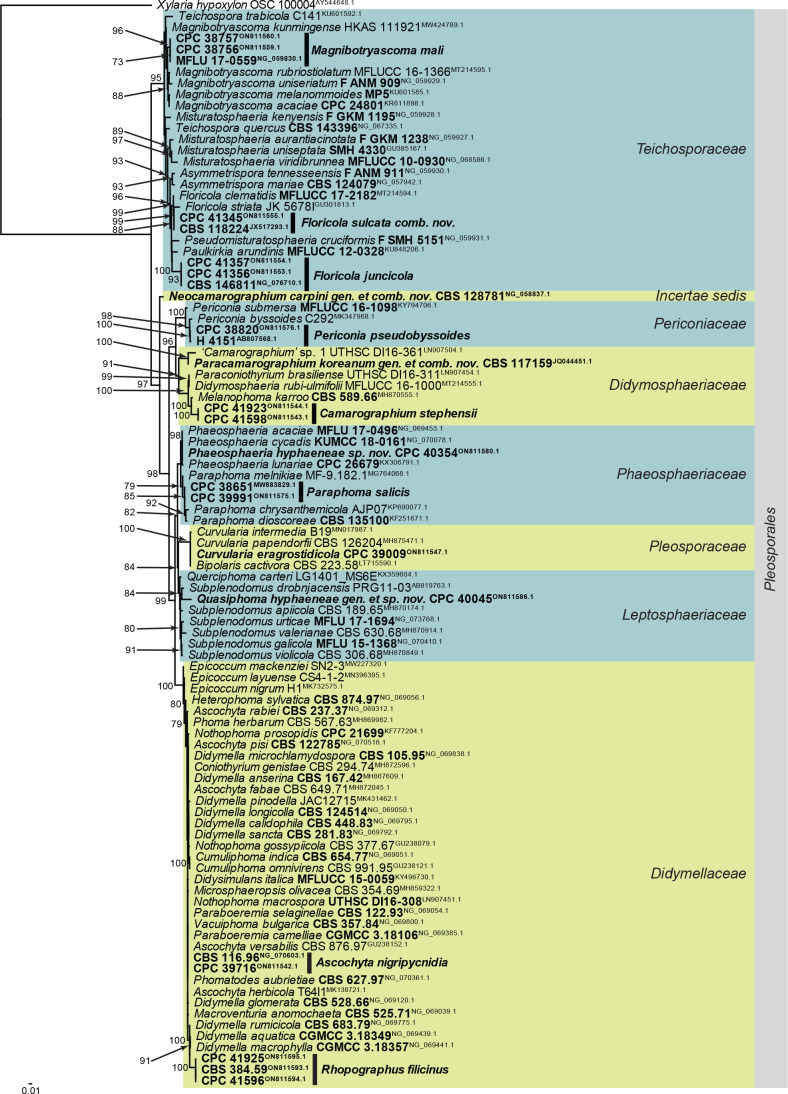
Fig. 8.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis of the Sordariomycetes (Xylariales) LSU nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Ramularia endophylla (culture CBS 113265; GenBank AY490776.2) and the species treated here are highlighted with coloured blocks and bold face. Accession numbers of sequence from material with a type status are also shown in bold face. Families and orders are indicated with coloured blocks to the right of the tree.
Species phylogenies: Specific phylogenetic analyses were run for selected species and the resulting phylogenies are discussed in the species notes where applicable.
The optimal identity thresholds to discriminate filamentous fungal species followed Vu et al. (2019), with secondary DNA barcodes generated where necessary (Stielow et al. 2015).
Taxonomy
Ascochyta nigripycnidia (Boerema et al.) Qian Chen & L. Cai, Stud. Mycol. 82: 187. 2015. Fig. 9.
Fig. 9.
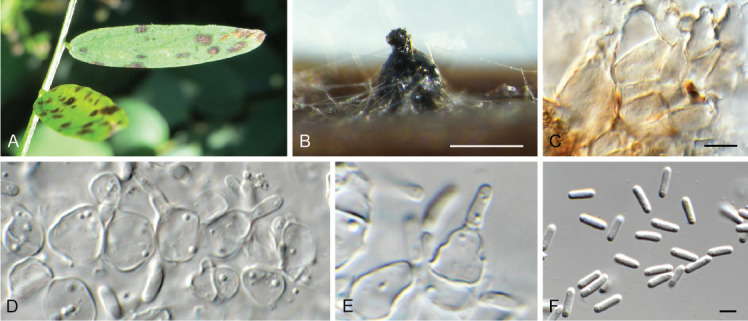
Ascochyta nigripycnidia (CPC 39716). A. Leaf spot symptoms on Vicia tenuifolia. B. Conidioma on PNA. C–E. Conidiogenous cells. F. Conidia. Scale bars: B = 300 μm, all others = 10 μm.
Basionym: Phoma nigripycnidia Boerema et al., Persoonia 16: 356. 1997.
Synonyms: Ascochyta nigripycnidiicola Ondřej, Biológia, Bratislava 23: 816. 1968.
Stagonosporopsis nigripycnidiicola (Ondřej) Boerema et al., Persoonia 16: 356. 1997.
Taxonomic lineage: Dothideomycetes, Pleosporales, Didymellaceae.
On PNA. Conidiomata pycnidial, solitary to aggregated, globose to subglobose, glabrous, black, 150–300 μm diam with prominent papillate neck giving rise to central ostiole; wall of 3–6 layers of brown textura angularis. Conidiogenous cells phialidic, hyaline, smooth-walled, ampulliform with prominent neck (2–4 μm long), 7–10 × 7–9 μm. Conidia cylindrical, hyaline, smooth- and thin-walled, aseptate, biguttulate, ends obtuse, (7–)8(–9) × 2(–2.5) μm.
Culture characteristics: Colonies flat, spreading, with moderate to abundant aerial mycelium and smooth, lobate margin, covering dish after 1 wk at 25 °C. On MEA, PDA and OA surface and reverse iron grey.
Material examined: Russia, Rostov region, Krasnosulinsky district, state natural wildlife area “Gornensky”, edge of ravine forest, on Vicia tenuifolia (Fabaceae), 27 Jun. 2020, T.S. Bulgakov, HPC 3374 = PC 073 = CBS H-24794 = LE F-332412, culture CPC 39716 = CBS 148249.
Notes: Ascochyta nigripycnidia (conidia 5.5–9 × 1.5–2 μm) was described from Vicia cracca in the Czech Republic (ex-type CBS 116.96) (Boerema et al. 1997, Chen et al. 2015), and is a good match for the present collection from Russia (Didymellaceae, Pleosporales; Fig. 2).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Ascochyta nigripycnidia [strain CBS 116.96, GenBank NR_135978.1; Identities = 485/486 (99 %), no gaps], Dothiorella gregaria [strain TS08-158-2, GenBank AB470899.1; Identities = 515/522 (99 %), one gap (0 %)], Phomatodes nebulosa [strain 21, GenBank MW580418.1; Identities = 514/521 (99 %), no gaps], and Ascochyta rabiei [voucher BAR-5, GenBank MK074843.1; Identities = 513/521 (98 %), no gaps]. Closest hits using the LSU sequence are Ascochyta nigripycnidia [strain CBS 116.96, GenBank NG_070603.1; Identities = 818/818 (100 %), no gaps], Vacuiphoma bulgarica [strain CBS 357.84, GenBank NG_069800.1; Identities = 818/818 (100 %), no gaps], Paraboeremia camelliae [strain CGMCC 3.18106, GenBank NG_069385.1; Identities = 818/818 (100 %), no gaps], and Paraboeremia litseae [strain CGMCC 3.18109, GenBank NG_069384.1; Identities = 818/818 (100 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Ascochyta nigripycnidia [strain CBS 116.96, GenBank MT018253.1; Identities = 592/596 (99 %), no gaps], Ascochyta koolunga [strain BRIP 70265, GenBank MN604922.1; Identities = 736/794 (93 %), no gaps], and Ascochyta lentis [as Didymella lentis; strain ATCC 96419, GenBank EU874862.1; Identities = 777/839 (93 %), no gaps]. No significant hits were obtained when the tef1 (first part) sequence was used in blastn and megablast searches.
Authors: P.W. Crous & J.Z. Groenewald
Ascocorticium sorbicola Crous & Declercq, sp. nov. MycoBank MB 844288. Fig. 10.
Fig. 10.
Ascocorticium sorbicola (CPC 40075). A. Conidiophores on SNA. B–D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Sorbus from which it was isolated.
Mycelium consisting of hyaline, smooth-walled, branched, septate, 1.5–2 μm diam hyphae. Conidiophores reduced to conidiogenous cells arising directly from hyphae, hyaline, smooth- and thin-walled, subulate, straight to flexuous, proliferating sympodially, forming a rachis in upper part, 20–42 × 2–3 μm, with multiple subdenticulate loci, slightly thickened and refractive, not darkened. Conidia solitary, hyaline, aseptate, smooth- and thin-walled, guttulate, subglobose, (3–) 3.5(–4) × 2.5(–3) μm; hilum slightly thickened, not darkened, 0.5 μm diam.
Culture characteristics: Colonies erumpent, spreading, surface folded, with sparse aerial mycelium and feathery, lobate margin, reaching 4 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface dirty white, reverse buff.
Typus: Belgium, Genk, Bokrijk, Het Wik, on leaves of Sorbus aucuparia (Rosaceae), 17 Sep. 2020, B. Declercq, HPC 3511 (holotype CBS H-24842, culture ex-type CPC 40075 = CBS 148303).
Notes: Ascocorticium anomalum is the type of Ascocorticium, an ascomycete species which forms white corticioid patches on wood or bark of gymnosperms and angiosperms, having acrodontium-like asexual morphs. Ascocorticium sorbicola is related to A. anomalum (conidia broadly ellipsoid, 3.5–5 × 3–3.5 μm), but has slightly smaller, subglobose conidia (Jülich & de Vries 1982). Phylogenetically (Fig. 4), the two species appear to not be congeneric, but as only a single isolate is available, we choose to describe it in Ascocorticium pending further collections.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest (distant) hits using the ITS sequence had highest similarity to Neohelicosporium krabiense [voucher HKAS 100725, GenBank NR_160380.1; Identities = 214/236 (91 %), two gaps (0 %)], Mikhtomia multicolor [strain SKA19, GenBank KJ021238.1; Identities = 219/242 (90 %), six gaps (2 %)], and Ascocorticium anomalum [strain CBS 874.71, GenBank MH860391.1; Identities = 277/311 (89 %), 12 gaps (3 %)]. Closest hits using the LSU sequence are Ascocorticium anomalum [strain CBS 874.71, GenBank MH872135.1; Identities = 757/806 (94 %), six gaps (0 %)], Sarcoleotia globosa [strain MBH52476, GenBank AY789428.1; Identities = 759/819 (93 %), six gaps (0 %)], and Glutinoglossum americanum [voucher ILLS 64444, GenBank KP690098.1; Identities = 762/823 (93 %), 10 gaps (1 %)]. No significant hits were obtained when the rpb2 and tef1 (second part) sequences were used in blastn and megablast searches.
Authors: P.W. Crous, J.Z. Groenewald & B. Declercq
Camarographium stephensii (Berk. & Broome) Bubák, Ber. dt. bot. Ges. 34: 306. 1916. Fig. 11.
Fig. 11.

Camarographium stephensii (CPC 41599). A. Immersed conidiomata. B. Conidial cirrhus. C. Conidiogenous cells. D–G. Conidiogenous cells giving rise to conidia. H. Conidia. Scale bars: B = 30 μm, all others = 10 μm.
Basionym: Hendersonia stephensii Berk. & Broome, Ann. Mag. Nat. Hist., Ser. 2 7: 95. 1851.
Synonym: Camarosporium stephensii (Berk. & Broome) Sacc., Syll. Fung. (Abellini) 3: 469. 1884.
Taxonomic lineage: Dothideomycetes, Pleosporales, Didymosphaeriaceae.
Conidiomata solitary or aggregated in linear eustromata, separate, globose, papillate, thick-walled, wall of hyaline to pale brown, thin-walled textura angularis; stromata linear, eye-shaped, immersed, subepidermal opening by irregular rupture through which ostioles appear, with thick-walled textura angularis, connecting pycnidia; elsewhere of hyaline, irregular pseudoparenchyma. Conidiophores reduced to conidiogenous cells, solitary, phialidic, enteroblastic, rarely percurrent, ampulliform to doliiform, hyaline, smooth, channel wide, collarette minute to prominent, lining the inner cavity of pycnidium, 7–10 × 4–8 μm. Conidia solitary, hyaline, becoming pale brown to brown, smooth to finely roughened, ellipsoid to irregularly so, or globose to obpyriform, apex obtuse, base flattened, 5 μm diam, distoseptate, thick-walled, with 3(–5) thick, transverse septa, and many thinner, longitudinal or oblique septa, (36–)45–50(–52) × (23–)25–28(–31) μm.
Typus: Netherlands, Utrecht Province, Bilthoven, on dead leaf fronds of Pteridium aquilinum (Hypolepidaceae), Jun. 2021, P.W. Crous, HPC 3632 (neotype designated here CBS H-24988, MBT 10007396, culture ex-neotype CPC 41598, CPC 41599 = CBS 149168).
Additional material examined: Netherlands, Utrecht Province, Lage Vuursche, on stem of Pteridium aquilinum, 13 Jun. 2021, P.W. Crous, HPC 3645, cultures CPC 41923, CPC 41924.
Notes: The genus Camarographium (Didymosphaeriaceae, Pleosporales), was introduced by Bubák (1916) with C. stephensii as type species. Because type material has presumably been lost, a neotype is designated here. Given the phylogenetic placement (Fig. 2) of C. stephensii, other species of Camarographium that are known from DNA sequence data need to be accommodated elsewhere (see below).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 41598 had highest similarity to Didymosphaeria rubi-ulmifolii [strain 8910, GenBank MK646046.1; Identities = 422/443 (95 %), no gaps], Paraconiothyrium brasiliense [strain A1202B, GenBank MT230470.1; Identities = 422/443 (95 %), no gaps], and Albifimbria verrucaria [as Myrothecium verrucaria; strain F0705, GenBank AB693919.1; Identities = 421/443 (95 %), no gaps]. The ITS sequences of CPC 41598 and 41923 are identical. Closest hits using the LSU sequence of CPC 41923 are Melanophoma karroo [strain CBS 589.66, GenBank MH870555.1; Identities = 790/805 (98 %), two gaps (0 %)], Paraconiothyrium brasiliense [strain UTHSC DI16-311, GenBank LN907454.1; Identities = 783/802 (98 %), one gap (0 %)], and Didymosphaeria rubi-ulmifolii [strain MFLUCC 16-1000, GenBank MT214555.1; Identities = 785/805 (98 %), two gaps (0 %)]. The LSU sequences of CPC 41598 and 41923 are identical. Closest hits using the tef1 (first part) sequence of CPC 41923 had distant similarity to Paraconiothyrium hakeae [strain CBS 142521, GenBank KY979892.1; Identities = 363/442 (82 %), 25 gaps (5 %)], Paraphaeosphaeria xanthorrhoeae [strain CBS 142164, GenBank KY979888.1; Identities = 255/299(85 %), five gaps (1 %)], and Paraphaeosphaeria parmeliae [strain CBS 131728, GenBank KP170679.1; Identities = 281/336 (84 %), 20 gaps (5 %)]. The tef1 sequences of CPC 41598 and 41923 are identical. Closest hits using the tub2 sequence of CPC 41923 had distant similarity to Paraconiothyrium hakeae [strain CBS 142521, GenBank KY979920.1; Identities = 458/535 (86 %), 14 gaps (2 %)], Paraconiothyrium variabile [strain CBS 504.84, GenBank JX496434.1; Identities = 389/455 (85 %), 14 gaps (3 %)], and Paraphaeosphaeria xanthorrhoeae [strain CBS 142164, GenBank KY979909.1; Identities = 441/538 (82 %), 17 gaps (3 %)]. The tub2 sequences of CPC 41598 and 41923 are identical.
Authors: P.W. Crous, J.Z. Groenewald, M. Starink-Willemse & A.L. van Iperen
Cercosporidium chaetomium (Cooke) Deighton, Mycol. Pap. 112: 27. 1967. Fig. 12.
Fig. 12.
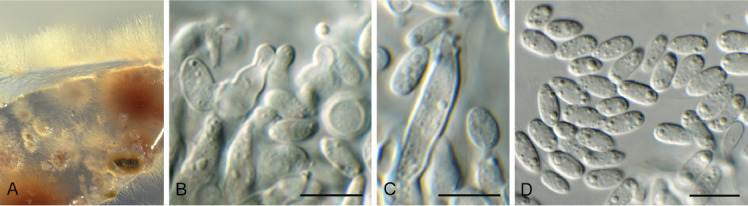
Cercosporidium chaetomium (CPC 39688). A. Leaf spots on Polygonum aviculare. B–D. Conidiophores and conidiogenous cells in culture. E, F. Conidiophores in vivo. G. Conidia. Scale bars = 10 μm.
Basionym: Cladosporium chaetomium Cooke, Grevillea 17(no. 83): 66. 1889.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Mycosphaerellaceae.
Leaf spots amphigenous, round, 2–3 mm diam, fair grey with red-purple margin. Conidiophores fasciculate, amphigenous, brown with minute brown stroma, subcylindrical, verruculose, 0–2-septate, unbranched, 10–25 × 4–6 μm. Conidiogenous cells terminal, integrated, brown, verruculose, subcylindrical, 10–15 × 3–4 μm; scars terminal, round, thickened, darkened, 1.5–2 μm diam. Conidia solitary, straight to slightly curved, obclavate, apex subobtuse, base obconically tapered to truncate hilum, pale brown, finely roughened, guttulate, (0–)3(–6)-septate, (20–)35–50(–60) × (3–)3.5(–4) μm; hila thickened, darkened, refractive, 2 μm diam. Conidia much longer in culture (–200 μm), and at times undergoing microcyclic conidiation.
Culture characteristics: Colonies erumpent, surface folded, with sparse aerial mycelium and feathery, lobate margin, reaching 7 mm diam after 7 d at 25 °C. On MEA surface smoke grey, reverse olivaceous grey with diffuse red pigment; on PDA surface and reverse olivaceous grey; on OA surface smoke grey.
Material examined: Russia, Rostov region, Krasnosulinsky district, state natural wildlife area “Gornensky”, roadside, on Polygonum aviculare (Polygonaceae), 13 Jun. 2020, T.S. Bulgakov, HPC 3312 = PC 054 = CBS H-24899 = LE F-332404, culture CPC 39688 = CBS 148457.
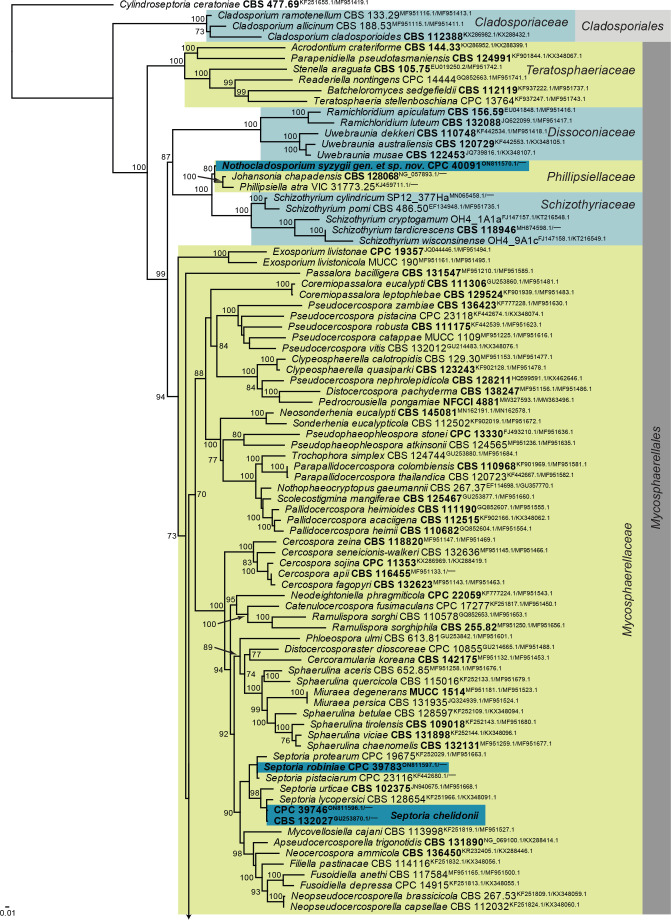
Notes: Cercosporidium chaetomium is the type species of the genus Cercosporidium, which was resurrected as distinct from Passalora by Videira et al. (2017). Cercosporidium is a common foliar pathogen, reported here from leaf spots on Polygonum aviculare in Russia. The genus belongs to Mycosphaerellaceae (Mycosphaerellales; Fig. 1 part 2) and the species is sister to Cercosporidium californicum and Cercosporidium miurae (Fig. 13).
Fig. 13.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 (Minh et al. 2020) of the Mycosphaerellales and Cladosporiales concatenated (LSU, rpb2) nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Cylindroseptoria ceratoniae (culture CBS 477.69; GenBank KF251655.1 and MF951419.1, respectively) and the species treated here are highlighted with coloured blocks and bold face. Accession numbers of sequence from material with a type status are also shown in bold face. Families and orders are indicated with coloured blocks to the right of the tree.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Cercosporidium chaetomium [strain CBS 142177, GenBank NR_156367.1; Identities = 470/474 (99 %), one gap (0 %)], Cercosporidium californicum [strain CPC 18389, GenBank NR_156239.1; Identities = 500/506 (99 %), one gap (0 %)], Clarohilum henningsii [strain IR-9, GenBank LC565143.1; Identities = 499/505 (99 %), no gaps], and Passalora dissiliens [as Phaeoramularia dissiliens; strain CBS 219.77, GenBank AF222835.1; Identities = 483/489 (99 %), no gaps]. Closest hits using the LSU sequence are Collarispora valgourgensis [strain CBS 129531, GenBank MH878078.1; Identities = 688/689 (99 %), no gaps], Paracercosporidium microsorum [as Mycosphaerella microsora; strain CBS 552.71, GenBank MH872022.1; Identities = 688/689 (99 %), no gaps], and Cercosporidium chaetomium [strain CBS 142177, GenBank NG_069527.1; Identities = 686/687 (99 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Cercosporidium chaetomium [strain CBS 142177, GenBank MF951474.1; Identities = 718/718 (100 %), no gaps], Cercosporidium californicum [strain CPC 18389, GenBank MF951471.1; Identities = 667/718 (93 %), no gaps], and Collarispora valgourgensis [strain CBS 125311, GenBank MF951480.1; Identities = 575/624 (92 %), no gaps].
Authors: P.W. Crous & J.Z. Groenewald
Coniella eucalyptorum (Crous & M.J. Wingf.) L.V. Alvarez & Crous, Stud. Mycol. 85: 15. 2016. Fig. 14.
Fig. 14.
Coniella eucalyptorum (CPC 39780). A. Conidiomata on OA. B–D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Basionym: Pilidiella eucalyptorum Crous & M.J. Wingf., Mycol. Res. 108: 296. 2004.
Taxonomic lineage: Sordariomycetes, Diaporthales, Schizo-parmaceae.
Description and illustration: Alvarez et al. (2016).
Material examined: South Africa, KwaZulu-Natal Province, Kwambonambi, on leaves of Eucalyptus benthamii (Myrtaceae), 8 Jun. 2020, J. Roux, HPC 3442, culture CPC 39780 = CBS 149182.
Notes: The genus Coniella includes saprobes, endophytes and plant pathogens (Alvarez et al. 2016). Of these, Coniella eucalyptorum is regarded as one of the more important foliar pathogens of Eucalyptus, and has been shown to cause significant defoliation on certain Eucalyptus species (Crous et al. 2019c). This is the first record of C. eucalyptorum occurring in South Africa. The species belongs to Schizoparmaceae (Diaporthales; Fig. 6).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Coniella eucalyptorum [strain CBS 112733, GenBank KX833548.1; Identities = 590/590 (100 %), no gaps], Coniella malaysiana [strain CBS 141598, GenBank NR_154820.1; Identities = 549/553 (99 %), no gaps], and Coniella eucalyptigena [strain CBS 139893, GenBank NR_137983.1; Identities = 558/574 (97 %), two gaps (0 %)]. Closest hits using the LSU sequence are Coniella eucalyptorum [strain CBS 115531, GenBank KX833382.1; Identities = 815/815 (100 %), no gaps], Coniella malaysiana [strain CBS 141598, GenBank NG_058965.1; Identities = 785/785 (100 %), no gaps], and Coniella eucalyptigena [strain CBS 139893, GenBank NG_058919.1; Identities = 799/811 (99 %), no gaps]. Closest hits using the tef1 (first part) sequence had highest similarity to Coniella eucalyptorum [strain CBS 114841, GenBank KX833646.1; Identities = 495/495 (100 %), no gaps], Coniella malaysiana [strain CBS 141598, GenBank KX833688.1; Identities = 408/465 (88 %), 14 gaps (3 %)], and Coniella granati [strain CBS 252.38, GenBank KX833681.1; Identities = 259/304 (85 %), 17 gaps (5 %)]. No significant hits were obtained when the tub2 sequence was used in blastn and megablast searches.
Authors: P.W. Crous, J.Z. Groenewald & J. Roux
Curvularia eragrostidicola Y.P. Tan & R.G. Shivas, MycoKeys 35: 14. 2018. Fig. 15.
Fig. 15.

Curvularia eragrostidicola (CPC 39009). A. Conidiophores on PNA. B–E. Conidiophores and conidiogenous cells giving rise to conidia. F. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Dothideomycetes, Pleosporales, Pleosporaceae.
Mycelium consisting of pale brown, smooth, branched, septate, 3–4 μm diam hyphae. Conidiophores solitary, erect, flexuous, subcylindrical, branched or not, pale brown to brown, smooth, geniculous-sinuous, 3–12-septate, 25–130 × 5–6 μm. Conidiogenous cells integrated, terminal and intercalary, polytretic, sympodial, pale to medium brown, 7–15 × 5–6 μm; scars darkened, thickened, 3–4 μm diam. Conidia ellipsoid-fusoid, widest in middle, tapering to obtuse ends, 3(–4)-distoseptate, septa not darkened, nor constricted, uniformly medium brown throughout, smooth-walled, swollen in second cell from apex, curved, hila thickened and darkened, 2–3 μm diam, (33–)36–38(–40) × (15–)16–17(–18) μm.
Culture characteristics: Colonies erumpent, spreading, with moderate to abundant aerial mycelium and smooth, even margin, covering dish after 2 wk at 25 °C. On MEA, PDA and OA surface olivaceous grey, reverse iron-grey.
Material examined: Namibia, Gobabeb Desert Namib Research Institute, Mirabib, on the dung of Procavia sp. (Procaviidae), 19 Nov. 2019, P.W. Crous, HPC 3110 = CBS H-24532, culture CPC 39009 = CBS 147069.
Notes: Curvularia eragrostidicola was described from inflorescences of Eragrostis pilosa collected in Australia (conidia (25–)26–30(–34) × (9–)13–15(–19) μm, 3-distoseptate; Tan et al. 2018), and this is the first record from Namibia. The species belongs to Pleosporaceae (Pleosporales; Fig. 2).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Curvularia eragrosticola [strain BRIP 12538, GenBank NR_158446.1; Identities = 557/561 (99 %), three gaps (0 %)], Curvularia papendorfii [strain ACSIKS_2102013, GenBank MN547549.1; Identities = 467/474 (99 %), one gap (0 %)], and Curvularia affinis [strain 14, GenBank FJ467358.1; Identities = 528/542 (97 %), six gaps (1 %)]. Closest hits using the LSU sequence are Curvularia papendorfii [strain CBS 126204, GenBank MH875471.1; Identities = 864/864 (100 %), no gaps], Bipolaris cactivora [strain CBS 223.58, GenBank LT715590.1; Identities = 864/864 (100 %), no gaps], and Curvularia intermedia [strain B19, GenBank MN017987.1; Identities = 863/864 (99 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Curvularia moringae [strain CPC 38873, GenBank MW173117.1; Identities = 852/900 (95 %), no gaps], Curvularia spicifera [strain C-6, GenBank KU133372.1; Identities = 760/812 (94 %), no gaps], and Curvularia crassiseptum [strain CBS 503.90, GenBank LT852473.1; Identities = 812/868 (94 %), no gaps]. Closest hits using the tef1 (second part) sequence had highest similarity to Curvularia eragrosticola [strain BRIP12538, GenBank MH433661.1; Identities = 860/869 (99 %), no gaps], Curvularia papendorfii [strain CBS 308.67, GenBank KM196594.1; Identities = 879/898 (98 %), one gap (0 %)], and Curvularia micropus [as Bipolaris micropus; strain UTHSC 07-1352, GenBank HE792957.1; Identities = 874/894 (98 %), one gap (0 %)].
Authors: P.W. Crous, J.Z. Groenewald, N. Yilmaz & M.J. Wingfield
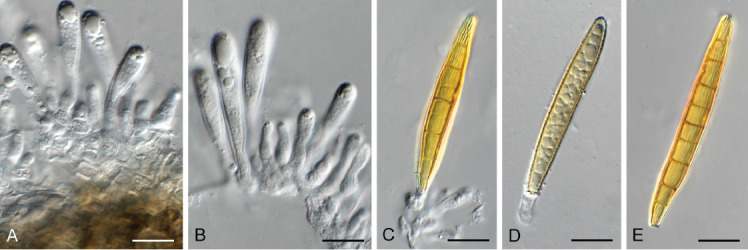
Dactylaria retrophylli Crous, sp. nov. MycoBank MB 844283. Fig. 16.
Fig. 16.
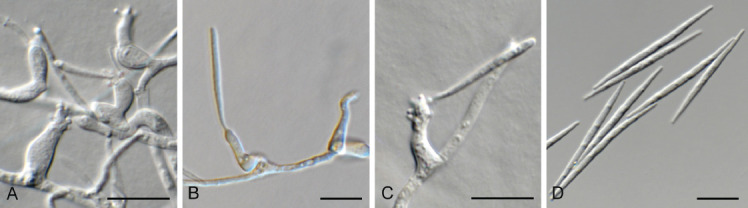
Dactylaria retrophylli (CPC 39510). A–C. Conidiogenous cells giving rise to conidia. D. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Sordariomycetes, Xylariales, incertae sedis.
Etymology: Name refers to the host genus Retrophyllum from which it was isolated.
Mycelium consisting of hyaline, septate, smooth-walled, branched, 1.5–2.5 μm diam hyphae. Conidiophores reduced to conidiogenous cells or with supporting cell, solitary, erect, geniculous-sinuous, subcylindrical, 6–20 × 2.5–3.5 μm; hyaline, smooth-walled, apex denticulate, 1–2 × 1 μm; not thickened nor darkened. Conidia solitary, dry, hyaline, smooth-walled, guttulate, medianly 1-septate, straight to narrowly fusoid, widest in middle, tapering towards both truncate ends, (26–)30–33(–37) × (1.5–)2 μm.
Culture characteristics: Colonies flat, spreading, surface folded, with sparse aerial mycelium and smooth, even margin, reaching 30 mm diam after 7 d at 25 °C. On MEA surface salmon, reverse ochreous; on PDA and OA surface and reverse pale luteous.
Typus: Colombia, Finca El Cedral, on leaves of Retrophyllum rospigliosii (Podocarpaceae), Feb. 2020, M.J. Wingfield, HPC 3260 (holotype CBS H-24817, culture ex-type CPC 39510 = CBS 148271).
Notes: Dactylaria (De Hoog 1985) is heterogeneous (Crous et al. 2016, 2017, 2018a), and the phylogenetic position of its type species, D. purpurella, remains unresolved. The present collection is tentatively named in Dactylaria, where it is phylogenetically distinct from other dactylaria-like taxa (Fig. 8). Morphologically, it is most similar to D. monticola in having medianly 1-septate, straight to narrowly fusoid conidia (30–37 × 1–1.5 μm) (Goh & Hyde 1997). Phylogenetically, it is related to D. monticola (CBS 188.95) in Xylariales, but distinct (Fig. 8).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Fusidium griseum [strain ICMP 15049, GenBank EF029217.1; Identities = 505/559 (90 %), 22 gaps (3 %)], Dactylaria acaciae [strain CPC 29771, GenBank KY173400.1; Identities = 507/563 (90 %), 18 gaps (3 %)], and Fusidium griseum [strain Trtsf08, GenBank GU479905.1; Identities = 504/563 (90 %), 21 gaps (3 %)]. Closest hits using the LSU sequence are Dactylaria monticola [strain P060, GenBank EU107289.1; Identities = 814/831 (98 %), three gaps (0 %)], Dactylaria zapatensis [strain P056, GenBank EU107287.1; Identities = 812/830 (98 %), six gaps (0 %)], and Dactylaria biseptata [strain P062, GenBank EU107288.1; Identities = 810/828 (98 %), six gaps (0 %)]. No Fusidium LSU sequences were available on GenBank for comparison.
Authors: P.W. Crous, J.Z. Groenewald, C.A. Rodas & M.J. Wingfield
Dactylellina miltoniae Crous, sp. nov. MycoBank MB 844280. Fig. 17.
Fig. 17.
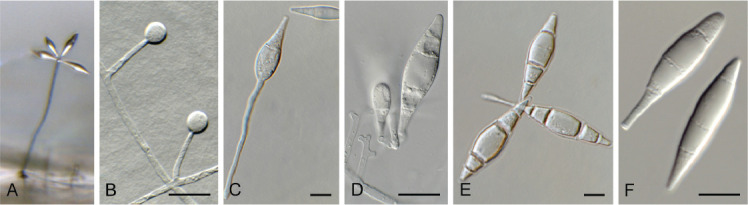
Dactylellina miltoniae (CPC 39508). A. Conidiophore giving rise to conidia. B. Adhesive knobs. C. Conidiogenous cell with attached conidium. D–F. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Orbiliomycetes, Orbiliales, Orbiliaceae.
Etymology: Name refers to the host genus Miltonia from which it was isolated.
Mycelium consisting of hyaline, septate, branched, 2–3 μm diam hyphae. Conidiophores erect, flexuous, subcylindrical, hyaline, smooth, septate, unbranched, 100–150 × 2–2.5 μm. Conidiogenous cells terminal, integrated, blastic, at times subdenticulate, giving rise to clusters of conidia. Conidia hyaline, smooth, guttulate, (2–)3–4-septate, ellipsoid to spindle-shaped or obconical, (23–)34–40(–47) × (5–)8–10(–11) μm. Adhesive knobs solitary, globose, hyaline, smooth, (4–)6–6.5 μm. Constricting rings, chlamydospores or sexual morph not observed.
Culture characteristics: Colonies flat, spreading, with sparse to moderate aerial mycelium and smooth, lobate margin, reaching 55 mm diam after 7 d at 25 °C. On MEA surface saffron, reverse ochreous; on PDA surface and reverse peach; on OA surface peach.
Typus: Colombia, Restrepo, on twigs of Miltonia clowesii (Orchidaceae), Feb. 2020, M.J. Wingfield, HPC 3270 (holotype CBS H-24816, culture ex-type CPC 39508 = CBS 148270).
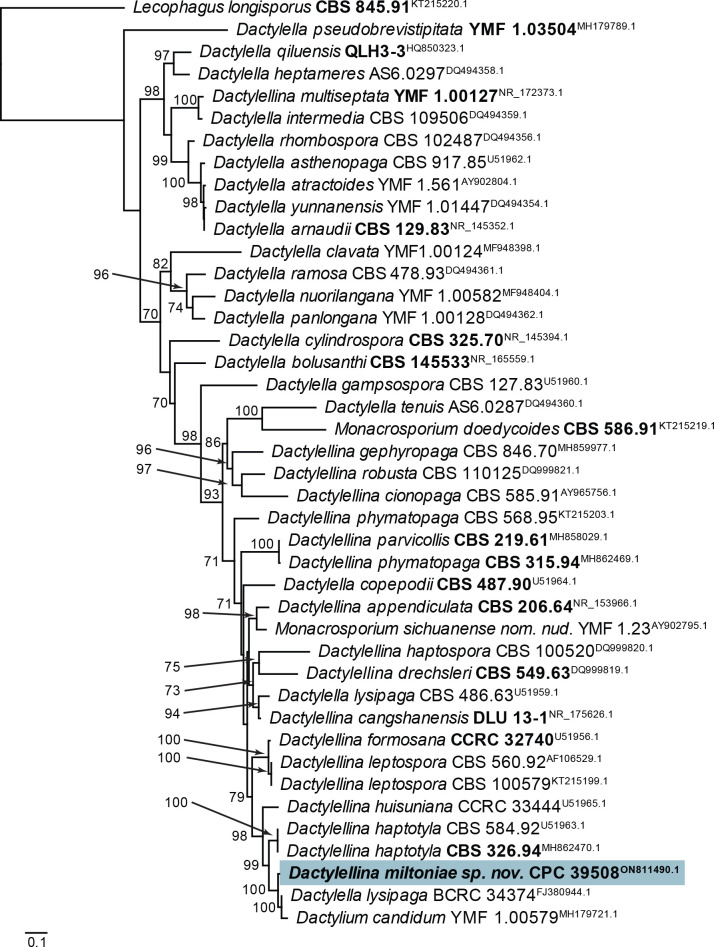
Notes: Dactylellina miltoniae was isolated from twigs of Miltonia clowesii, and is characterised by small adhesive knobs, and 2–4-septate conidia on flexuous conidiophores. It is morphologically similar to D. haptotyla (distinct in that it has small adhesive knobs), and D. yuannanensis (distinct in that it lacks con-constricting rings). Phylogenetically, it clusters close to Clade C of the D. haptotyla s.l. complex, as defined by Baral et al. (2020) in Orbiliaceae (Orbiliales; Fig. 5). It is closely allied to Dactylella lysipaga and Dactylium candidum (Fig. 18).
Fig. 18.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 (Minh et al. 2020) of the Dactylella/Dactylellina ITS nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Lecophagus longisporus (culture CBS 845.91; GenBank KT215220.1) and the species treated here is highlighted with bold face. Accession numbers of sequence from material with a type status are also shown in bold face.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Dactylium candidum [strain 1.00579, GenBank MH179721.1; Identities = 537/545 (99 %), no gaps], Dactylella lysipaga [strain BCRC 34374, GenBank FJ380944.1; Identities = 540/549 (98 %), one gap (0 %)], and Dactylellina haptotyla [as Monacrosporium haptotylum; strain XJ03-96-1, GenBank DQ999827.1; Identities = 497/519 (96 %), nine gaps (1 %)]. Closest hits using the LSU sequence are Stilbella bucidae [strain CBS 694.96, GenBank MH874229.1; Identities = 730/737 (99 %), no gaps], Dactylellina haptotyla [as Monacrosporium sclerohyphum; strain KMF1.00041, GenBank AY965762.1; Identities = 730/737 (99 %), no gaps], and Dactylium candidum [strain YMF1.36, GenBank AY902801.1; Identities = 730/737 (99 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Dactylellina haptotyla [strain XJ03-96-1, GenBank DQ999804.1; Identities = 741/779 (95 %), one gap (0 %)], Dactylellina haptotyla [as Monacrosporium haptotylum; strain SQ95-2, GenBank AY773441.1; Identities = 695/737 (94 %), one gap (0 %)], and Dactylellina appendiculata [strain CBS 206.64, GenBank DQ358229.1; Identities = 653/761 (86 %), one gap (0 %)]. Closest hits using the tef1 (second part) sequence had highest similarity to Dactylella lysipaga [GenBank AY695066.1; Identities = 652/713 (91 %), no gaps], Orbilia ellipsospora [as Monacrosporium ellipsosporum; GenBank AY695064.1; Identities = 645/713 (90 %), no gaps], and Dactylellina drechsleri [as Monacrosporium drechsleri; GenBank AY695062.1; Identities = 645/713 (90 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald, C.A. Rodas & M.J. Wingfield
Dothiora viticola (Laich & Stchigel) Crous, comb. nov. MycoBank MB 844304.
Basionym: Hormonema viticola Laich & Stchigel, Persoonia 34: 229. 2015.
Taxonomic lineage: Dothideomycetes, Dothideales, Dothideaceae.
Description and illustration: See Crous et al. (2015).
Typus: Spain, Canary Islands, Lanzarote, La Geria, 28.9764; -13.6917, from fruit (grapes) of Vitis vinifera cv. Malvasia, Aug. 2009, coll. F. Laich, isol. S.S. Gonzaìlez-Gonzaìlez & F. Laich (holotype CBS H-22115, cultures ex-type CBS 140676 = FMR 13040 = L9D-17).
Notes: The Dothideales was treated by Hongsanan et al. (2020b), and four families recognised, namely Dothideaceae, Neocelosporiaceae, Saccotheciaceae and Zalariaceae (also see Fig. 1 part 1). However, many of the genera in these families remain poorly understood due to the lack of DNA data related to their respective type species.
Dothiora viticola appears similar to other species of Dothideaceae in that cultures produce endoconidia. Dothiora viticola appears to have a wider host range and distribution than originally assumed, also occurring in grassland rhizospheres (Spain), orchids (Chile), as an endophyte of Dactylis glomerata, and associated with beetle galleries on walnut (Juglans regia) twigs in the USA. Also see the phylogenetic trees in Crous et al. (2015) for the placement of this species.
Authors: P.W. Crous & J.Z. Groenewald
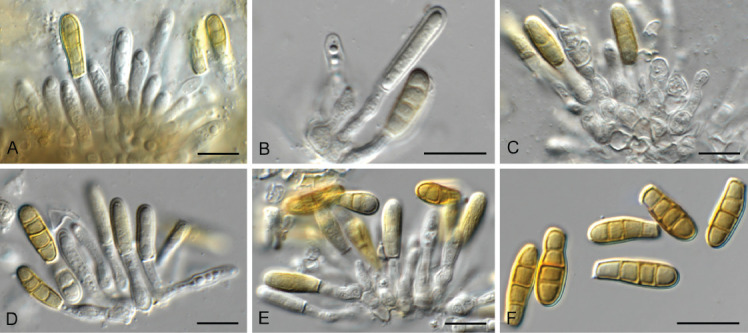
Elsinoe corni Jenkins & Bitanc., J. Wash. Acad. Sci. 38: 362. 1948. Fig. 19.
Fig. 19.
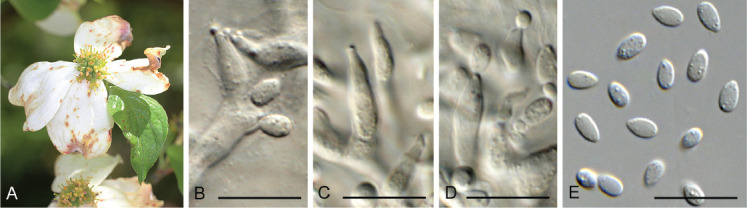
Elsinoe corni (CBS 41728). A. Disease spots on Cornus florida. B–D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Dothideomycetes, Myriangiales, Elsinoaceae.
Description and illustration: Jenkins & Bitancourt (1948).
Typus: USA, North Carolina, Highlands, MACON Co., on Cornus florida (Cornaceae), 5 Sep. 1947, J.A. Stevenson (holotype BPI 679270); North Carolina, Charlotte, 35.078519, -81.001553, on leaves of Cornus florida, 17 Apr. 2021, A. Loyd (epitype designated here, CBS H-24990, MBT 10007399, culture ex-epitype CPC 41728 = CBS 148184).
Notes: Elsinoe causes scab (or anthracnose) disease on numerous plant hosts (Fan et al. 2017). Elsinoe corni is the causal agent of spot anthracnose of flowering dogwood (Cornus florida) in the USA, causing small, circular spots on bracts, petioles, peduncles, stems and leaves. The pathogen was originally described by Jenkins & Bitancourt (1948) from Cornus florida collected in North Carolina. The present collection provides good material to serve as epitype to resolve the phylogeny of the species (Fig. 1 part 1).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Elsinoe fawcettii [strain CBS 139.25, GenBank MH854816.1; Identities = 570/594 (96 %), six gaps (1 %)], Elsinoe australis [voucher 1629, GenBank MG956760.1; Identities = 570/594 (96 %), six gaps (1 %)], and Elsinoe diospyri [strain CBS 223.50, GenBank MH856596.1; Identities = 537/558 (96 %), six gaps (1 %)]. Closest hits using the LSU sequence are Elsinoe populi [strain CBS 289.64, GenBank KX887036.1; Identities = 734/736 (99 %), no gaps], Elsinoe diospyri [strain CBS 223.50, GenBank KX886973.1; Identities = 733/736 (99 %), no gaps], and Elsinoe tiliae [strain CBS 350.73, GenBank KX887059.1; Identities = 732/736 (99 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Elsinoe populi [strain CBS 289.64, GenBank KX887154.1; Identities = 700/744 (94 %), no gaps], Elsinoe diospyri [strain CBS 223.50, GenBank KX887093.1; Identities = 699/744 (94 %), no gaps], and Elsinoe caleae [strain CBS 221.50, GenBank KX887088.1; Identities = 699/744 (94 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald & A. Loyd
Elsinoe parthenocissi Jenkins & Bitanc., Phytopathology 32: 424. 1942. Fig. 20.
Fig. 20.
Elsinoe parthenocissi (CPC 38770). A. Sporodochia on SNA. B, C. Conidiogenous cells giving rise to conidia. D. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Dothideomycetes, Myriangiales, Elsinoaceae.
Conidiomata sporodochial, erumpent, hyaline to pale brown, 200–300 μm diam. Conidiophores subcylindrical, hyaline to pale brown, smooth, 0–1-septate, at times reduced to conidiogenous cells. Conidiogenous cells integrated, terminal, subcylindrical to doliiform, hyaline, smooth, with age somewhat pigmented, mono- to polyphialidic, 9–17 × 4–5 μm. Conidia hyaline, granular, aseptate, ellipsoid, apex obtuse, tapering towards base with truncate hilum, (6–)7–8(–9) × (3–)4(–4.5) μm.
Culture characteristics: Colonies erumpent, spreading, surface folded, with sparse aerial mycelium and smooth, lobate margin, reaching 7 mm diam after 2 wk at 25 °C. On MEA surface and reverse sienna to umber; on PDA surface rust, reverse umber; on OA surface scarlet.
Material examined: New Zealand, Auckland, Devan Port, on Parthenocissus quinquefolia (Vitaceae), 2019, C. Inglis, CBS H-24481, culture CPC 38770 = T19_06384B = CBS 146969.
Notes: Elsinoe parthenocissi (Fig. 1 part 1) was described from symptomatic leaves, stems and petioles of Parthenocissus quinquefolia collected in New Hampshire, USA (BPI 680610 type) (Jenkins & Bitancourt 1942). Elsinoe parthenocissi is the only species known from Parthenocissus, and has previously been reported from New Zealand by Dingley (1965). Only the asexual morph was collected in the present study, so the species identification remains unconfirmed, as it could also be an undescribed species of Elsinoe occurring on this host in New Zealand.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Elsinoe diospyri [strain CBS 223.50, GenBank NR_148135.1; Identities = 519/528 (98 %), four gaps (0 %)], Elsinoe caleae [strain CBS 221.50, GenBank NR_148131.1; Identities = 518/527 (98 %), three gaps (0 %)], and Elsinoe tiliae [strain CBS 350.73, GenBank KX887296.1; Identities = 515/526 (98 %), four gaps (0 %)]. Closest hits using the LSU sequence are Elsinoe tiliae [strain CBS 350.73, GenBank KX887059.1; Identities = 732/736 (99 %), no gaps], Elsinoe populi [strain CBS 289.64, GenBank KX887036.1; Identities = 732/736 (99 %), no gaps], and Elsinoe perseae [strain CBS 288.64, GenBank KX887020.1; Identities = 731/736 (99 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Elsinoe populi [strain CBS 289.64, GenBank KX887154.1; Identities = 689/744 (93 %), no gaps], Elsinoe diospyri [strain CBS 223.50, GenBank KX887093.1; Identities = 688/744 (92 %), no gaps], and Elsinoe caleae [strain CBS 221.50, GenBank KX887088.1; Identities = 688/744 (92 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald & R. Thangavel
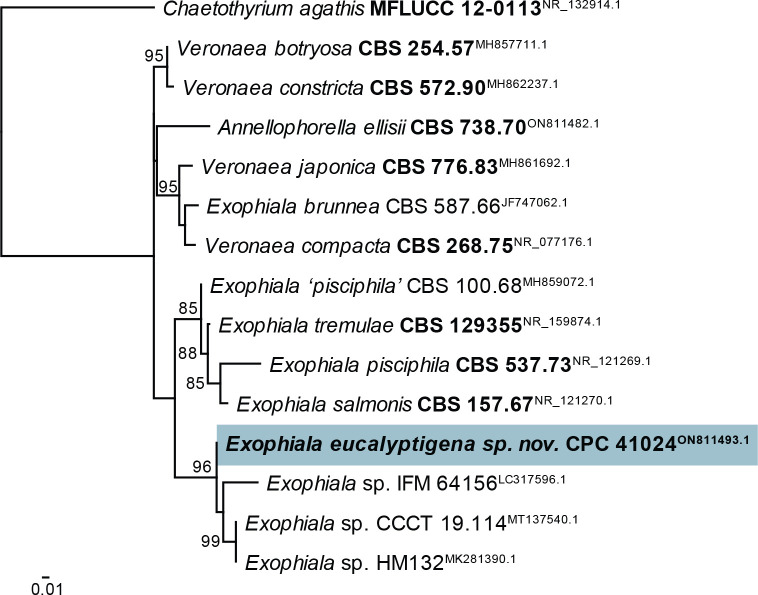
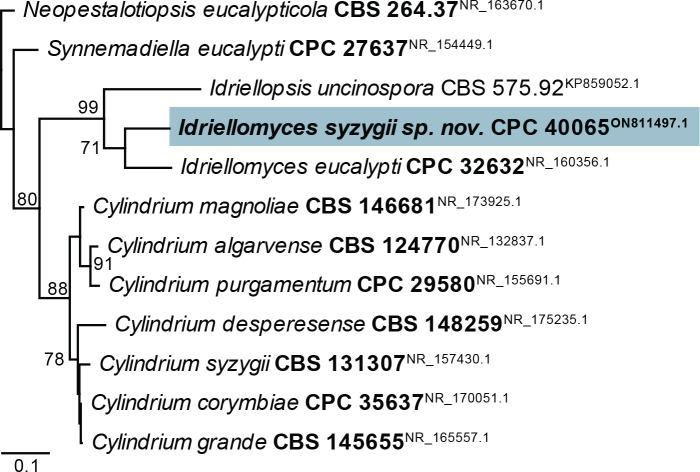
Exophiala eucalyptigena Crous, sp. nov. MycoBank MB 844281. Fig. 21.
Fig. 21.
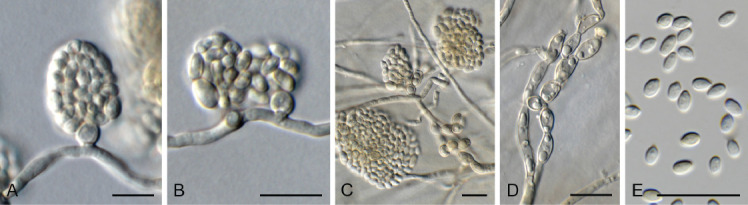
Exophiala eucalyptigena (CPC 41024). A–D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Eurotiomycetes, Chaetothyriales, Herpotrichiellaceae.
Etymology: Name refers to the host genus Eucalyptus from which it was isolated.
Mycelium consisting of pale brown, smooth-walled, guttulate, septate, branched, 1.5–3 μm diam hyphae. Hyphal cells becoming constricted at septa, and more ellipsoid closer to conidiogenous region. Conidiogenous cells solitary or in clusters, ampulliform with elongated neck, with numerous percurrent proliferations, pale to medium brown, smooth, base subglobose, 3–4 × 4–5 μm; neck cylindrical, 2–4 × 1.5–2 μm; rarely reduced to cylindrical, erect conidiogenous loci, direct on hyphae. Conidia solitary, aseptate, ellipsoid, smooth-walled, pale brown, guttulate, apex obtuse, tapering to narrowly truncate hilum, 0.5 μm diam, (3–)3.5–4(–5) × 2(–2.5) μm.
Culture characteristics: Colonies flat, spreading, surface folded, with sparse aerial mycelium and smooth, lobate margin, reaching 5 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface olivaceous grey, reverse iron-grey.
Typus: Australia, Australian Capital Territory, on dead leaves of Eucalyptus viminalis subsp. viminalis (Myrtaceae) supporting Idolothrips spectrum population, 17 Jan. 2015, A. Wells & L.A. Mound (holotype CBS H-24819, culture ex-type CPC 41024 = CBS 148273).
Notes: Exophiala eucalyptigena clusters among species of Exophiala s. str. (Crous et al. 2020a; Figs 3, 22). It is morphologically distinct from other species of Exophiala in that the conidiogenous cells are frequently arranged in clusters, and have elongated, cylindrical necks.
Fig. 22.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 (Minh et al. 2020) of the Exophiala ITS nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Chaetothyrium agathis (culture MFLUCC 12-0113; GenBank NR_132914.1) and the species treated here is highlighted with bold face. Accession numbers of sequence from material with a type status are also shown in bold face.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Exophiala pisciphila [strain CBS 100.68, GenBank MH859072.1; Identities = 571/624 (92 %), 16 gaps (2 %)], Veronaea botryosa [strain MFLUCC 11-0072, GenBank MG922570.1; Identities = 571/624 (92 %), 18 gaps (2 %)], and Exophiala tremulae [strain CBS 129355, GenBank NR_159874.1; Identities = 570/624 (91 %), 16 gaps (2 %)]. Closest hits using the LSU sequence are Exophiala equina [strain CBS 128222, GenBank MH876297.1; Identities = 807/813 (99 %), no gaps], Annellophorella ellisii [strain CBS 738.70, GenBank MH871721.1; Identities = 805/813 (99 %), no gaps], and Helicoarctatus thailandicus [strain MFLUCC 18-0332, GenBank MK559870.1; Identities = 804/813 (99 %), no gaps]. Closest hits using the tef1 (first part) sequence had highest similarity to Exophiala salmonis [strain AFTOL-ID 671, GenBank EF413612.1; Identities = 258/288 (90 %), six gaps (2 %)], Exophiala pisciphila [strain AFTOL-ID 669, GenBank DQ840567.1; Identities = 268/320 (84 %), 16 gaps (5 %)], and Exophiala abietophila [voucher HGUP-R300, GenBank MK887139.1; Identities = 239/283 (84 %), 11 gaps (3 %)]. Closest hits using the tub2 sequence had highest similarity to Veronaea botryosa [strain 608911, GenBank MN477320.1; Identities = 326/414 (79 %), 16 gaps (3 %)], Exophiala salmonis [strain CBS 120274, GenBank KF928562.1; Identities = 304/387 (79 %), 18 gaps (4 %)], and Exophiala oligosperma [strain CBS 124085, GenBank KF928552.1; Identities = 287/364 (79 %), 15 gaps (4 %)].
Authors: P.W. Crous, J.Z. Groenewald, L. Mound, A. Wells & C.C. Linde
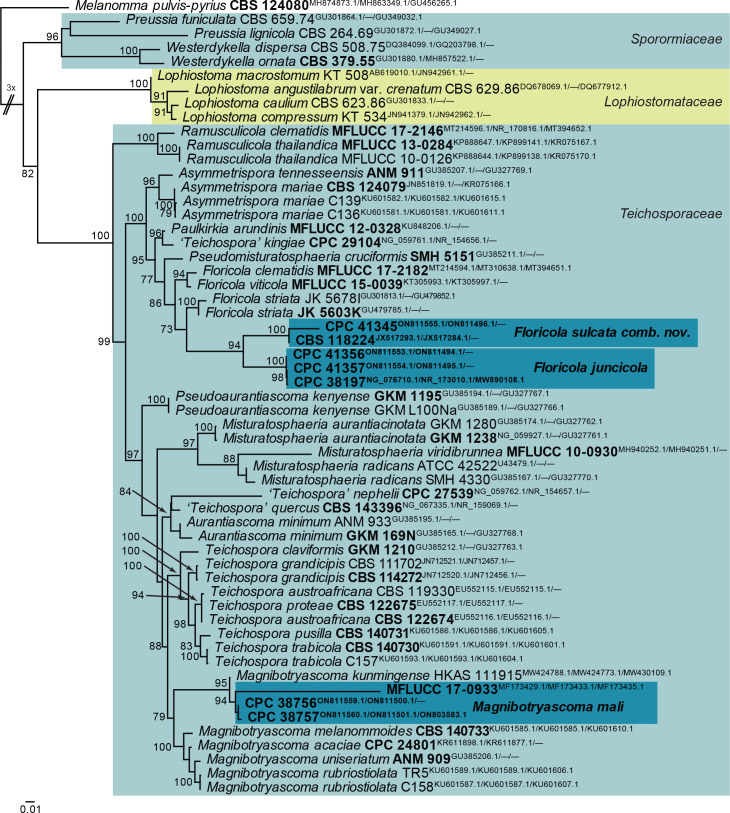
Floricola juncicola Crous & R.K. Schumach., Fungal Syst. Evol. 7: 289. 2021. Fig. 23.
Fig. 23.
Floricola juncicola (CPC 41357). A–C. Conidiogenous cells giving rise to conidia. D, E. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Dothideomycetes, Pleosporales, Teichosporaceae.
Description and illustration: Crous et al. (2021b).
Material examined: Netherlands, Gelderland, Hoge Veluwe, Deelense Wasch, 52°05’37’’N, 5°51’17’’E, on dead culm of Juncus effusus (Juncaceae), 1 Mar. 2021, E.R. Osieck, HPC 3609 = WI-29/#4220, cultures CPC 41356 = CBS 148318, CPC 41357 = CBS 148287.
Notes: Floricola juncicola was described from dead culms of Juncus collected in France (Crous et al. 2021b), and found here to also be common on Juncus spp. in the Netherlands. The species belongs to Teichosporaceae (Pleosporales; Figs 2, 24).
Fig. 24.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 (Minh et al. 2020) of the Teichosporaceae concatenated (LSU, ITS, tef1) nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Melanomma pulvis-pyrius (culture CBS 124080; GenBank MH874873.1, MH863349.1, GU456265.1, respectively) and the species treated here are highlighted with coloured blocks and bold face. Accession numbers of sequence from material with a type status are also shown in bold face. Families are indicated with coloured blocks to the right of the tree.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 41356 had highest similarity to Floricola juncicola [strain CBS 146811, GenBank NR_173010.1; Identities = 433/434 (99 %), no gaps], Teichospora viticola [strain MFLUCC 15-0039, GenBank NR_154624.1; Identities = 367/385 (95 %), two gaps (0 %)], and Teichospora kingiae [strain CPC 29104, GenBank NR_154656.1; Identities = 362/386 (94 %), six gaps (1 %)]. The ITS sequences of CPC 41356 and 41357 are identical (434/434 bp). Closest hits using the LSU sequence of CPC 41356 are Floricola juncicola [strain CBS 146811, GenBank NG_076710.1; Identities = 831/832 (99 %), one gap (0 %)], Paulkirkia arundinis [strain MFLUCC 12-0328, GenBank KU848206.1; Identities = 810/829 (98 %), two gaps (0 %)], and Floricola clematidis [strain MFLUCC 17-2182, GenBank MT214594.1; Identities = 809/829 (98 %), two gaps (0 %)]. The LSU sequences of CPC 41356 and 41357 are identical (814/814 bp). Closest hits using the rpb2 sequence of CPC 41356 had highest similarity to Floricola juncicola [strain CPC 38197, GenBank MW890063.1; Identities = 645/647 (99 %), no gaps], Teichospora mariae [strain C136, GenBank KU601595.1; Identities = 742/858 (86 %), two gaps (0 %)], and Teichospora striata [strain JK 5678I, GenBank GU371758.1; Identities = 712/878 (81 %), seven gaps (0 %)]. The rpb2 sequences of CPC 41356 and 41357 are almost identical (842/845 bp, no gaps]. Closest hits using the tef1 (first part) sequence of CPC 41356 had highest similarity to Floricola juncicola [strain CPC 38197, GenBank MW890092.1; Identities = 502/503 (99 %), no gaps], Astragalicola amorpha [strain C227a, GenBank MF795842.1; Identities = 257/320 (80 %), 30 gaps (9 %)], and Setophoma longinqua [strain LC6593, GenBank MK525069.1; Identities = 253/319 (79 %), 22 gaps (6 %)]. The tef1 sequences of CPC 41356 and 41357 differ with a single nucleotide (502/503 bp, no gaps).
Authors: P.W. Crous, J.Z. Groenewald & E.R. Osieck
Floricola sulcata (Marinc. et al.) Crous & Osieck, comb. nov. MycoBank MB 844284. Fig. 25.
Fig. 25.
Floricola sulcata (CPC 41345). A–E. Conidiogenous cells giving rise to conidia. F. Conidia. Scale bars = 10 μm.
Basionym: Sclerostagonospora sulcata Marinc. et al., Sydowia 69: 252. 2017.
Taxonomic lineage: Dothideomycetes, Pleosporales, Teichosporaceae.
Conidiomata pycnidial, solitary, globose, dark brown with central ostiole, 150–250 μm diam; wall of 3–6 layers of brown textura angularis. Conidiophores subcylindrical, hyaline, smooth-walled, 0–1-septate, branched at base or not, 7–20 × 3–5 μm. Conidiogenous cells integrated, terminal, subcylindrical, hyaline, smooth-walled, 7–15 × 2.5–4 μm, proliferating percurrently at apex. Conidia solitary, subcylindrical, apex obtuse, at times slightly clavate, tapering to truncate hilum, 2–3 μm diam, with marginal frill, golden brown, roughened with striations along its entire length, (1–)3-septate, (11–)13–14(–15) × 4(–6) μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 30 mm diam after 2 wk at 25 °C. On MEA surface olivaceous grey, reverse smoke grey; on PDA surface olivaceous grey, reverse iron grey with diffuse red pigment; on OA surface olivaceous grey.
Material examined: Netherlands, Overijssel Province, Engbertsdijksvenen, near Kloosterhaar, 52°28’57’’N, 6°40’02’’E, 17 m a.s.l., on dead culm of Juncus effusus (Juncaceae), 9 Mar. 2021, E.R. Osieck, HPC 3611 = WI-30/#4226 = CBS H-24832, culture CPC 41345 = CBS 148286.
Notes: Sclerostagonospora sulcata was described from culm litter of Restio subverticellatus collected in South Africa (Krisai-Greilhuber et al. 2017), with conidia being brown, striate, 3-septate, (13–)14–15(–17) × (4–)5–6(–7) μm. This taxon appears to be better accommodated in the genus Floricola (Phukhamsakda et al. 2020, Crous et al. 2021b; Teichosporaceae, Pleosporales, Figs 2, 24). This is the fourth species of the genus collected from monocotyledons (and the third from Juncus). The other two Floricola species were described from Clematis vitalba and Vitis vinifera.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Sclerostagonospora sulcata [as Sclerostagonospora sp.; ex-type strain CBS 118224, GenBank JX517284.1; Identities = 487/488 (99 %), no gaps], Teichospora viticola [strain MFLUCC 15-0039, GenBank NR_154624.1; Identities = 395/411 (96 %), two gaps (0 %)], and “Floricola” clematidis [as Teichospora sp. CP-2020b; strain MFLUCC 17-2182, GenBank MT310638.1; Identities = 394/411 (96 %), two gaps (0 %)]. Closest hits using the LSU sequence are Sclerostagonospora sulcata [ex-type strain CBS 118224, GenBank JX517293.1; Identities = 815/815 (100 %), no gaps], “Floricola” clematidis [strain MFLUCC 17-2182, GenBank MT214594.1; Identities = 814/815 (99 %), no gaps], and Paulkirkia arundinis [strain MFLUCC 12-0328, GenBank KU848206.1; Identities = 806/815 (99 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to “Floricola” juncicola [strain CPC 38197, GenBank MW890063.1; Identities = 583/648 (90 %), two gaps (0 %)], Teichospora striata [strain JK 5678I, GenBank GU371758.1; Identities = 713/848 (84 %), two gaps (0 %)], and Teichospora quercus [strain CBS 143396, GenBank MH108010.1; Identities = 660/837 (79 %), 22 gaps (2 %)]. Closest hits using the tef1 (first part) sequence had highest similarity to “Floricola” juncicola [strain CPC 38197, GenBank MW890092.1; Identities = 348/392 (89 %), 24 gaps (6 %)], Teichospora trabicola [strain C141, GenBank KU601603.1; Identities = 297/362 (82 %), 17 gaps (4 %)], and Teichospora melanommoides [strain MP5, GenBank KU601610.1; Identities = 289/352 (82 %), 14 gaps (3 %)].
Authors: P.W. Crous, J.Z. Groenewald & E.R. Osieck
Idriellomyces syzygii Crous, sp. nov. MycoBank MB 844285. Fig. 26.
Fig. 26.
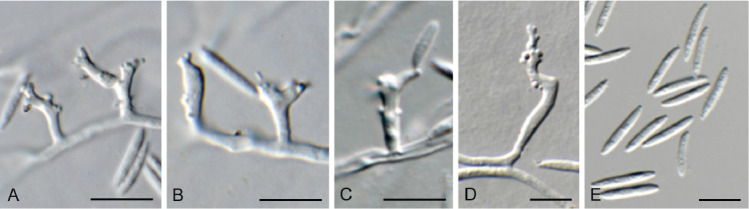
Idriellomyces syzygii (CPC 40065). A–D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Sordariomycetes, Xylariales, Phlogicylindriaceae.
Etymology: Name refers to the host genus Syzygium from which it was isolated.
Mycelium consisting of hyaline, smooth-walled, septate, branched, 1.5–2 μm diam hyphae, forming hyphal coils. Conidiophores reduced to conidiogenous cells, arising directly from superficial mycelium, straight to geniculous-sinuous, hyaline, smooth- and thin-walled, 3–20 × 2–3 μm; apical part forming a rachis with truncate, denticulate loci, 0.5–1 × 0.5 μm, not thickened nor darkened. Conidia solitary, hyaline, smooth-walled, aseptate, falcate, apex subobtuse, base truncate, 0.5 μm diam, curved more prominently on dorsiventral side than inner plane, guttulate, (7–)8–10(–11) × (1.5–)2 μm.
Culture characteristics: Colonies flat, spreading, with sparse aerial mycelium and smooth, lobate margin, reaching 20 mm diam after 2 wk at 25 °C. On MEA surface and reverse buff; on PDA and OA surface and reverse dirty white.
Typus: South Africa, KwaZulu-Natal Province, near Mozambique border, on leaves of Syzygium cordatum (Myrtaceae), 19 Oct. 2017, M.J. Wingfield, HPC 3503 (holotype CBS H-24797, culture ex-type CPC 40065 = CBS 148252).
Notes: Idriellomyces is based on I. eucalypti, known from Eucalyptus obliqua leaves collected in Australia (Crous et al. 2018c). Idriellomyces syzygii also occurs on Myrtaceae, namely Syzygium cordatum leaves collected in South Africa, where it was mycophylic on a Meliola species. Idriellomyces syzygii differs from I. eucalypti in that the scars on the conidiogenous cells are more pronounced, denticulate, the conidiophores are hyaline, and it has larger conidia than those of I. eucalypti, (5–)6.5–7(–8) × 1.5(–2) μm (Crous et al. 2018c). Based on the phylogenetic trees generated here, the species is best accommodated in Idriellomyces (Phlogicylindriaceae, Xylariales; Figs 8, 27).
Fig. 27.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 (Minh et al. 2020) of the Cylindrium ITS nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Neopestalotiopsis eucalypticola (culture CBS 264.37; GenBank NR_163670.1) and the species treated here is highlighted with bold face. Accession numbers of sequence from material with a type status are also shown in bold face.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Idriellopsis uncinospora [strain CBS 575.92, GenBank KP859052.1; Identities = 370/414 (89 %), 14 gaps (3 %)], Idriellomyces eucalypti [strain CPC 32632, GenBank NR_160356.1; Identities = 462/534 (87 %), 30 gaps (5 %)], and Cylindrium elongatum [strain CBS 115974, GenBank KM231853.1; Identities = 463/545 (85 %), 31 gaps (5 %)]. Closest hits using the LSU sequence are Idriellomyces eucalypti [strain CPC 32632, GenBank NG_066413.1; Identities = 786/804 (98 %), two gaps (0 %)], Castanediella cagnizarii [strain CBS 101043, GenBank KP858988.1; Identities = 770/804 (96 %), two gaps (0 %)], and Castanediella tereticornis [strain CBS 145068, GenBank NG_068600.1; Identities = 769/804 (96 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald & M.J. Wingfield
Leptopeltis litigiosa (Desm.) L. Holm & K. Holm, Bot. Notiser 130: 221. 1977. Fig. 28.
Fig. 28.
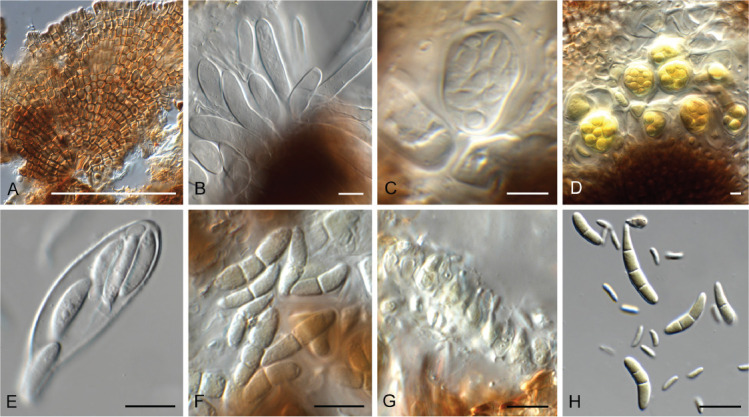
Leptopeltis litigiosa (CPC 41928). A. Superficial wall of thyrothecium. B–E. Asci. F. Ascospores. G. Conidiogenous cells. H. Ascospores and conidia. Scale bars: A = 125 μm, all others = 10 μm.
Basionym: Leptostroma litigiosum Desm., Annls Sci. Nat., Bot., sér. 2 19: 338. 1843.
Synonyms: Dothithyrella litigiosa (Desm.) Höhn., Annls mycol. 16: 171. 1918.
Leptostroma litigiosum Desm., Annls Sci. Nat., Bot., sér. 2 19: 338. 1843.
Leptostroma litigiosum var. exasperatum Berk., in Hooker, Bot. Antarct. Voy. Erebus Terror 1839-1843, II, Fl. Nov.-Zeal.: 193. 1855.
Microthyrium litigiosum (Desm.) Sacc., Michelia 1: 496. 1879.
Leptothyrium litigiosum (Desm.) Sacc., Michelia 2: 113. 1880.
Pycnothyrium litigiosum (Desm.) Died., Annls mycol. 11: 175. 1913. (asexual morph)
Taxonomic lineage: Leotiomycetes, incertae sedis, Leptopeltidaceae.
Thyrothecia subcuticular, brown, round, 100–250 μm diam, becoming confluent and forming larger crusts, opening by irregular rupture; surface of radiating rows of brown textura angularis; margin even, undulate. Paraphyses intermingled among asci, hyaline, smooth, septate, 2–2.5 μm diam, disintegrating at maturity. Asci hyaline, smooth, subcylindrical, apex subobtuse, somewhat tapered, slightly curved, 30–50 × 8–10 μm, base truncate to bluntly rounded, unitunicate, apex slightly thickened, at times straining slightly in Melzer’s (unclear reaction). Ascospores tri to multiseriate, hyaline, smooth, guttulate, fusoid-ellipsoid, slightly curved, ends subobtuse, granular, (0–)1–3-septate, (10–)12–15(–20) × (3–) 3.5–4 μm. Pycnothyria intermingled among thyrothecia, round, brown, 150–250 μm diam, with central ostiole. Conidiophores reduced to conidiogenous cells lining basal cavity, ampulliform, hyaline, smooth, 3–5 × 3–4 μm. Conidia solitary, hyaline, smooth, subcylindrical, apex obtuse, base tapering to truncate hilum, straight to curved, aseptate, granular, 4–7 × 1.5–2 μm. Ascospores in thyrothecia give rise to conidia via budding.
Typus: France, J.B.H.B. Desmazières, on Pteridium aquilinum (Hypolepidaceae), Moug. & Nestl., Stirpes Crypt. 673 (UPS), designated as lectotype here, MBT 10007403. Netherlands, Utrecht Province, Bilthoven, on dead leaf fronds of Pteridium aquilinum, Jun. 2021, P.W. Crous, HPC 3645 (epitype designated here CBS H-24991, MBT 10007404, culture ex-epitype CPC 41927, 41928 = CBS 149171).
Notes: The genus Leptopeltis was introduced based on L. filicina (Leptopeltidaceae), which we regard as a distinct genus, sensu Hongsanan et al. (2020a), and reject the suggested synonymy with Fouragea filicina (based on Opegrapha filicina 1845), as the basionym of L. filicina is older (1834). However, as molecular data for the type species L. filicina is lacking, its phylogeny remains unresolved. For the present, we have assigned this taxon to Leptopeltidaceae with no clear ordinal affinity in Leotiomycetes (Fig. 4)
Leptopeltis litigiosa is common on dead leaf fronds of Pteridium aquilinum, and often occurs intermixed with L. pteridis (= L. aquilina), which has larger, elongated black ascomata with a non-radiate shield, and slightly smaller ascospores, 12–15 × 3–4 μm (Holm & Holm 1977). Holm & Holm (1977) regarded asci to be unitunicate, while Eriksson (1981) stated that they were bitunicate. Although difficult to determine, in the present study asci appeared more unitunicate than bitunicate. Furthermore, based on single ascospore cultures, the asexual morph of L. litigiosa could be resolved as coelomycetous.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Pragmopora cf. pini [voucher G.M. 2019-05-19.1, GenBank MN547972.1; Identities = 473/525 (90 %), nine gaps (1 %)], Claussenomyces sp. keyed as caeruleomarginatus [voucher G.M. 2019-06-13.3, GenBank MN238834.1; Identities = 466/515 (90 %), 10 gaps (1 %)], and Pragmopora cf. bacillifera [voucher G.M. 2019-04-30.1, GenBank MK900749.1; Identities = 462/515 (90 %), nine gaps (1 %)]. Closest hits using the LSU sequence are Pallidophorina paarla [strain CBS 120877, GenBank NG_068607.1; Identities = 688/708 (97 %), two gaps (0 %)], Ramoconidiophora euphorbiae [strain CBS 141018, GenBank NG_068606.1; Identities = 684/708 (97 %), two gaps (0 %)], and Capturomyces luteus [strain GLMC 1842, GenBank MK314603.1; Identities = 683/708 (96 %), two gaps (0%)].
Authors: P.W. Crous, J.Z. Groenewald, M. Starink-Willemse & A.L. van Iperen
Macgarvieomyces juncicola (MacGarvie) Klaubauf et al., Stud. Mycol. 79: 107. 2014. Fig. 29.
Fig. 29.

Macgarvieomyces juncicola (CPC 40815). A. Conidiophores on SNA. B–E. Conidiophores and conidiogenous cells giving rise to conidia. Scale bars = 10 μm.
Basionym: Pyricularia juncicola MacGarvie, Scientific Proc. R. Dublin Soc., Ser. B 2(no. 16): 155. 1968.
Taxonomic lineage: Sordariomycetes, Magnaporthales, Pyriculariaceae.
Description and illustration: Klaubauf et al. (2014).
Material examined: Netherlands, Utrecht Province, Nieuw Wulven, near Houten, 52°02’45’’N, 5°10’34’’E, 1.5 m a.s.l., on dead culm of Juncus effusus (Juncaceae), 8 Jan. 2021, E.R. Osieck, HPC 3564 = WI-10/#4195, culture CPC 40815 = CBS 148264.
Notes: Macgarvieomyces juncicola is known from culms of Juncus effusus in Ireland and the Netherlands (Klaubauf et al. 2014), and the present isolate represents a new collection for the species (Fig. 6).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Macgarvieomyces juncicola [strain CBS 610.82, GenBank KM484855.1; Identities = 461/461 (100 %), no gaps], Dactylaria junci [strain P17, GenBank AY265321.1; Identities = 498/508 (98 %), no gaps], and Macgarvieomyces luzulae [strain CBS 145042, GenBank MK442591.1; Identities = 495/532 (93 %), 16 gaps (3 %)]. Closest hits using the LSU sequence are Macgarvieomyces juncicola [strain CBS 610.82, GenBank KM009153.1; Identities = 815/815 (100 %), no gaps], Macgarvieomyces luzulae [strain CBS 145042, GenBank MK442531.1; Identities = 805/815 (99 %), one gap (0 %)], and Macgarvieomyces borealis [strain CBS 461.65, GenBank MH870310.1; Identities = 803/815 (99 %), one gap (0 %)]. Closest hits using the actA sequence had highest similarity to Macgarvieomyces juncicola [strain CBS 610.82, GenBank KM485171.1; Identities = 317/317 (100 %), no gaps], Macgarvieomyces luzulae [strain CBS 145042, GenBank MK442635.1; Identities = 634/724 (88 %), 31 gaps (4 %)], and Pseudopyricularia bothriochloae [strain CBS 136427, GenBank KY905700.1; Identities = 598/713 (84 %), 45 gaps (6 %)]. Closest hits using the cmdA sequence had highest similarity to Macgarvieomyces juncicola [strain CBS 610.82, GenBank KM485240.1; Identities = 477/477 (100 %), no gaps], and Macgarvieomyces luzulae [strain CPC 31555, GenBank MG934520.1; Identities = 384/461 (83 %), 19 gaps (4 %)]. Closest hits using the rpb1 sequence had highest similarity to Macgarvieomyces juncicola [strain CBS 610.82, GenBank KM485071.1; Identities = 749/749 (100 %), no gaps], Macgarvieomyces luzulae [strain CPC 31571, GenBank MG934471.1; Identities = 555/605 (92 %), no gaps], and Pyricularia borealis [strain CBS 461.65, GenBank KM009186.1; Identities = 652/716 (91 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald & E.R. Osieck
Magnibotryascoma mali Phukhams. et al., Fungal Diversity 87: 105. 2017. Fig. 30.
Fig. 30.
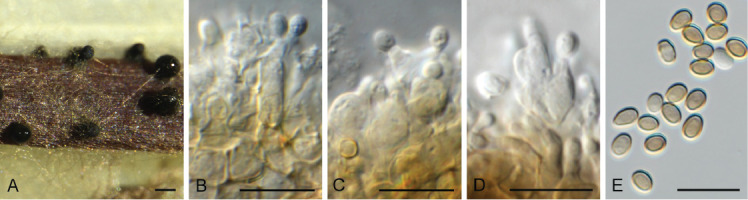
Magnibotryascoma mali (CPC 38756). A. Conidiomata on PNA. B–D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars: A = 200 μm, all others = 10 μm.
Taxonomic lineage: Dothideomycetes, Pleosporales, Teichosporaceae.
Conidiomata solitary, erumpent, globose, brown, 150–200 μm diam, with central ostiole; wall of 6–8 layers of brown textura angularis. Conidiophores lining the inner cavity, reduced to conidiogenous cells or with a basal supporting cell, branched or not. Conidiogenous cells hyaline, smooth, phialidic with prominent periclinal thickening, ampulliform to subcylindrical, 5–10 × 3–5 μm. Conidia solitary, aseptate, thick-walled, guttulate, initially hyaline, smooth, becoming golden brown, ellipsoid to subovoid, 3–4 × 2.5–3 μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and lobate, smooth margin, reaching 50 mm diam after 2 wk at 25 °C. On MEA surface and reverse isabelline; on PDA surface isabelline, reverse brown vinaceous; on OA surface isabelline.
Material examined: New Zealand, Tuaranga port, on Metrosideros sp. (Myrtaceae), 22 Aug. 2019, L. Rabbidge, CBS H-24440, culture CPC 38757 = T19_05741B = CBS 146778; Tauranga port, on Metrosideros sp., 2019, L. Rabbidge, CBS H-24506, culture CPC 38756 = T19_05741A = CBS 147001.
Notes: Magnibotryascoma mali was recently described on Malus halliana collected in China (conidia 3–5 × 2.3–3.8 μm, oval to broadly obovoid, hyaline when young, become reddish brown at maturity, aseptate, smooth-walled; Hyde et al. 2017). The New Zealand collection is phylogenetically similar and has overlapping conidial dimensions. Based on the blast results and the phylogenetic trees (Fig. 2, 24), our cultures can best be accommodated in this species. The long branch for the ex-type culture of this species (Fig. 24) is most likely due to an issue with the ex-type ITS sequence. Based on blast searches using the ex-type sequence, the best hit is with Pseudochaetosphaeronema kunmingense (Pleosporales), which contradicts the placement of this strain based on the other loci.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 38757 had highest similarity to Magnibotryascoma sp. DNW-2020a [strain HKAS 111915, GenBank MW424773.1; Identities = 547/549 (99 %), one gap (0 %)], Teichospora rubriostiolata [strain CBS 140734, GenBank NR_154634.1; Identities = 552/605 (91 %), 20 gaps (3 %)], and Teichospora melanommoides [strain CBS 140733, GenBank NR_154632.1; Identities = 554/607 (91 %), 25 gaps (4 %)]. The ITS sequences of CPC 38756 and 38757 are identical (591/591 bp). Closest hits using the LSU sequence of CPC 38757 are Magnibotryascoma mali [voucher MFLU 17-0559, GenBank NG_059830.1; Identities = 817/817 (100 %), no gaps], Magnibotryascoma sp. DNW-2020a [strain HKAS 111921, GenBank MW424789.1; Identities = 857/865 (99 %), no gaps], and Teichospora acaciae [strain CBS 140000, GenBank MH878675.1; Identities = 772/780 (99 %), two gaps (0 %)]. The LSU sequences of CPC 38756 and 38757 are identical (850/850 bp). Closest hits using the tef1 (first part) sequence of CPC 38757 had highest similarity to Teichospora rubriostiolata [strain C158x, GenBank KU601608.1; Identities = 405/462 (88 %), eight gaps (1 %)], Teichospora melanommoides [strain MP5, GenBank KU601610.1; Identities = 405/463 (87 %), 13 gaps (2 %)], and Teichospora pusilla [strain C140, GenBank KU601605.1; Identities = 403/467 (86 %), 17 gaps (3 %)]. The tef1 sequences of CPC 38756 and 38757 are identical (492/492 bp). Closest hits using the tef1 (second part) sequence of CPC 38757 had highest similarity to Magnibotryascoma mali [strain MFLUCC 17-0933, GenBank MF173435.1; Identities = 899/907 (99 %), no gaps], Ramusculicola thailandica [strain MFLUCC 13-0284, GenBank KR075167.1; Identities = 785/849 (92 %), no gaps], and Rhytidhysteron rufulum [voucher MFLU 18-2190, GenBank MK360087.1; Identities = 791/857 (92 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald & R. Thangavel
Microcera lichenicola Crous & Boers, sp. nov. MycoBank MB 844286. Fig. 31.
Fig. 31.
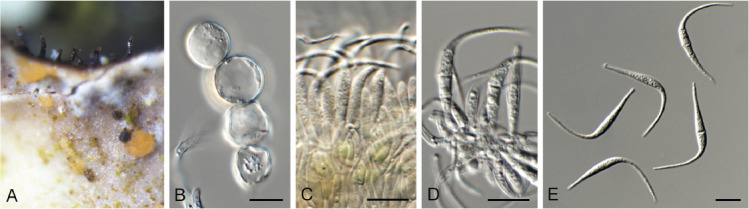
Microcera lichenicola (CPC 41114). A. Discoloured thalli of Parmelia sulcata with orange sporodochia. B. Chlamydospores. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Sordariomycetes, Hypocreales, Nectriaceae.
Etymology: Name refers to the lichen hosts from which it was isolated.
Associated with discoloured thalli of Parmelia sulcata. Mycelium consisting of hyaline, smooth-walled, branched, septate, 2–3 μm diam hyphae. Conidiomata sporodochial, orange on host, consisting of tightly aggregated conidiophores that are reduced to conidiogenous cells or with a short stipe, giving rise to 1–4 conidiogenous cells. Conidiogenous cells subulate, tapering towards apex, flexuous, monophialidic with periclinal thickening and minute non-flared collarette, 10–20 × 2–3 μm. Conidia hyaline, smooth-walled, guttulate, (0–)3-septate, distinctly curved and twisted, apex attenuated, subobtuse, dorsiventrally curved, basal cell attenuated to subobtuse, non-foot-shaped to slightly notched basal cell, (35–)40–45(–55) × 3(–3.5) μm.
Culture characteristics: Colonies flat, spreading, surface folded, with sparse aerial mycelium and smooth, lobate margin, reaching 22 mm diam after 14 d at 25 °C. On MEA surface orange, reverse orange with patches of ochreous; on PDA and OA surface and reverse orange. Cultures sterile, forming chains of hyaline, subglobose to globose chlamydospores, 6–15 μm diam.
Typus: Netherlands, Friesland Province, Ameland, Ballum, on Parmelia sulcata (Parmeliaceae), 21 Feb. 2021, J. Boers, HPC 3594 (holotype CBS H-24992, culture ex-type CPC 41114 = CBS 149169).
Material examined of Microcera sp.: Netherlands, Friesland Province, Ameland, Ballum, on Physcia tenella (Physciaceae), 21 Feb. 2021, J. Boers, HPC 3596, culture CPC 41230 = CBS 148313; North Holland Province, Ameland, Ballum, on Parmelia sulcata (Parmeliaceae), 21 Feb. 2021, J. Boers, HPC 3594, culture CPC 41115 = CBS 149170.
Notes: Although species of Microcera are usually pathogens of scale insects (Gräfenhan et al. 2011, Crous et al. 2021c), this is the second species described from a lichen thallus. Microcera lichenicola is phylogenetically (Figs. 7, 32) and morphologically similar to M. physciae (on Physcia tenella), but distinct in that the latter has shorter conidia, (20–)24–26(–33) × 3(–3.5) μm (Crous et al. 2021d). Although the ITS and LSU sequences of CPC 41114 and 41230 are highly similar, their tub2 sequences are only 96 % similar. Unfortunately we were unable to generate a complete dataset for both strains and therefore prefer not to apply the name Microcera lichenicola to CPC 41230 pending the collection of more strains from this host (family).
Fig. 32.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 (Minh et al. 2020) of the Microcera concatenated (ITS, rpb1, rpb2, tub2) nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Macroconia leptosphaeriae (culture CBS 100001; GenBank HQ897810.1, KM232255.1, HQ728164.1, KM232097.1, respectively) and the species treated here is highlighted with bold face. Accession numbers of sequence from material with a type status are also shown in bold face.
The ITS sequence of CPC 41114 differs with a single nucleotide from the ex-type sequence of Microcera physciae [strain CBS 148283, GenBank NR_175225.1; Identities = 496/497 (99 %); based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 41114 had highest similarity to “Fusarium sp.” [strain F-267,620, GenBank EU860076.1; Identities = 544/545 (99 %), one gap (0 %)], Microcera larvarum [strain ICMP 5444, GenBank MT107902.1; Identities = 534/546 (98 %), three gaps (0 %)], and Cosmospora aurantiicola [strain F-251,305, GenBank EU860061.1; Identities = 533/545 (98 %), three gaps (0 %)]. The LSU sequence of CPC 41114 differs with a single nucleotide from the ex-type sequence of Microcera physciae [strain CBS 148283, GenBank OK663766.1; Identities = 813/814 (99 %); closest hits using the LSU sequence of CPC 41114 are Microcera rubra [strain CBS 638.76, GenBank NG_058100.1; Identities = 806/816 (99 %), no gaps], Microcera larvarum [strain CBS 738.79, GenBank KM231701.1; Identities = 806/816 (99 %), no gaps], and Microcera coccophila [strain MAFF 241482, GenBank KC291787.1; Identities = 781/793 (98 %), no gaps]. The tub2 sequence of CPC 41114 is 97 % similar to the ex-type sequence of Microcera physciae strain CBS 148283, GenBank OK651208.1; Identities = 510/526 (97 %), two gaps (0 %)]; closest hits using the tub2 sequence of CPC 41114 had highest similarity to “Fusarium sp.” [strain F-267,620, GenBank EU860029.1; Identities = 527/542 (97 %), one gap (0 %)], Microcera larvarum [as Fusarium larvarum var. larvarum; strain F-266,784, GenBank EU860024.1; Identities = 470/542 (86 %), eight gaps (1 %)], and Microcera coccophila [strain MAFF 241482, GenBank KC291936.1; Identities = 449/515 (87 %), eight gaps (1 %)].
The ITS sequences of CPC 41230 and 41114 differ at two nucleotide positions (543/545 bp identical) while the LSU sequences of CPC 41230 and 41114 differ at three nucleotide positions (798/801 bp identical). The rpb1 sequence of CPC 41230 is 96 % similar to the ex-type sequence of Microcera physciae [strain CBS 148283, GenBank OK651153.1; Identities = 710/738 (96 %), no gaps]; closest hits using the rpb1 sequence of CPC 41230 had highest similarity to Microcera coccophila [strain MAFF 241482, GenBank KC291895.1; Identities = 631/688 (92 %), no gaps], Microcera larvarum [strain A.R. 4580, GenBank KC291894.1; Identities = 627/688 (91 %), no gaps], and Microcera rubra [strain CBS 638.76, GenBank KM232253.1; Identities = 654/724(90 %), no gaps]. The rpb2 sequence of CPC 41230 is 96 % similar to the ex-type sequence of Microcera physciae [strain CBS 148283, GenBank OK651168.1; Identities = 845/883 (96 %), one gap (0 %)]; closest hits using the rpb2 sequence of CPC 41230 had highest similarity to Microcera sp. [strain NRRL 26790, GenBank JX171636.1; Identities = 832/863 (96 %), no gaps], Microcera larvarum [strain NRRL 20473, GenBank JX171587.1; Identities = 775/861 (90 %), no gaps], and Microcera rubra [strain CBS 638.76, GenBank KM232388.1; Identities = 773/859 (90 %), no gaps]. The tef1 (first part) sequence of CPC 41230 is 94 % similar to the ex-type sequence of Microcera physciae [strain CBS 148283, GenBank OK651190.1; Identities = 434/464 (94 %), four gaps (0 %)]; closest hits using the tef1 (first part) sequence of CPC 41230 had highest similarity to “Fusarium sp.” [strain NRRL 20473, GenBank JF740695.1; Identities = 379/465 (82 %), 19 gaps (3 %)], Microcera larvarum [strain CBS 738.79, GenBank KM231957.1; Identities = 334/412 (81 %), 19 gaps (4 %)], and Microcera coccophila [strain ZJUP0179, GenBank MN614420.1; Identities = 221/255 (87 %), two gaps (0 %)]. The tub2 sequences of CPC 41230 and 41114 are 96 % similar (505/525 bp, including three gaps).
Authors: P.W. Crous, J.Z. Groenewald & J. Boers
Mycodiella eucalypti Crous, Persoonia 37: 337. 2016. Fig. 33.
Fig. 33.
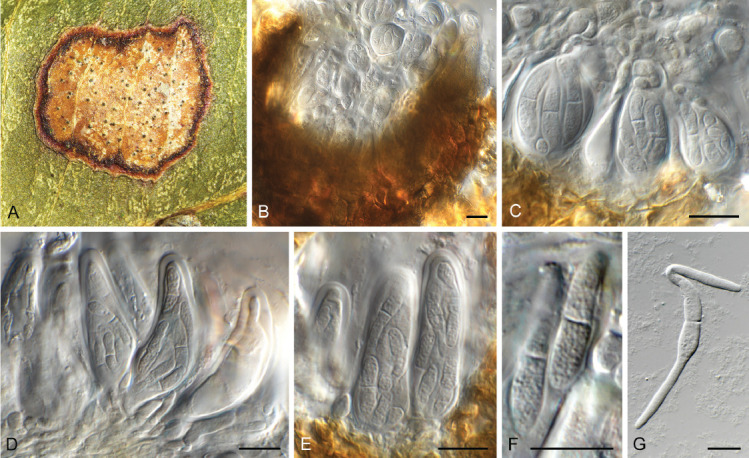
Mycodiella eucalypti (CPC 38962). A. Leaf spot with ascomata. B. Broken ascoma with asci. C–E. Asci. F. Ascospores. G. Germinating ascospore. Scale bars = 10 μm.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Mycosphaerellaceae.
Leaf spots amphigenous, irregular, brown with raised red-brown border, 2–5 mm diam. Ascomata amphigenous, erumpent, globose, brown, 100–130 μm diam, with central periphysate ostiole, 10–15 μm diam; wall of 3–4 layers of brown textura angularis. Asci fasciculate, 8-spored, bitunicate, obovoid, curved to straight, with apical ocular chamber, 35–45 × 13–16 μm. Ascospores multiseriate, hyaline, smooth, guttulate, medianly 1-septate, thin-walled, not constricted at septum, subcylindrical to narrowly obovoid, widest in upper part of apical cell, (15–) 17–18(–20) × (3–)3.5–4 μm; ascospores germinating from both ends, with germ tubes parallel to the long axis, flexuous, becoming constricted at septum, 6–7 μm diam, remaining hyaline.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium and lobate, even margin, reaching 15 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface olivaceous grey, reverse iron-grey.
Material examined: South Africa, Northern Province, Nelspruit, Buffelskloof Nature Reserve, on leaf litter of Syzygium cordatum (Myrtaceae), Nov. 2018, P.W. Crous, HPC 3148 = CBS H-24498, culture CPC 38962 = CBS 146986.
Notes: Mycodiella eucalypti (on Eucalyptus diversicolor, Australia, ascospores (11–)12–13(–15) × (2.5–)3(–3.5) μm; Crous et al. 2016) was originally described as having smaller ascospores than the present collection. However, the two collections are phylogenetically closely related (Fig. 1 part 2, 13), suggesting that the South African collection is best treated as M. eucalypti until more strains of the species become available for comparison.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Mycodiella eucalypti [strain CPC 29458, GenBank NR_155408.1; Identities = 526/536 (98 %), gap (0 %)], Mycodiella sumatrensis [strain CBS 118499, GenBank NR_111803.1; Identities = 455/468 (97 %), two gaps (0 %)], and Mycosphaerella polygoni-cuspidati [strain IMI 401968, GenBank LC146384.1; Identities = 494/512 (96 %), two gaps (0 %)]. Closest hits using the LSU sequence are Mycodiella sumatrensis [strain CBS 118499, GenBank NG_042737.1; Identities = 712/714 (99 %), one gap (0 %)], Mycodiella eucalypti [strain CPC 29458, GenBank NG_059747.1; Identities = 964/971 (99 %), no gaps], and Mycosphaerella laricis-leptolepidis [strain MAFF 410081, GenBank JX901862.1; Identities = 705/713 (99 %), no gaps]. Closest hits using the actA sequence had highest similarity to Mycodiella eucalypti [strain CPC 29525, GenBank KY173566.1; Identities = 491/497 (99 %), no gaps], Mycodiella sumatrensis [strain CBS 118499, GenBank KF903498.1; Identities = 514/522 (98 %), no gaps], and Mycosphaerella laricis-leptolepidis [strain MAFF 410234, GenBank JX902112.1; Identities = 509/541 (94 %), three gaps (0 %)]. Closest hits using the cmdA sequence had highest similarity to Mycodiella sumatrensis [strain CBS 118502, GenBank KF902552.1; Identities = 352/365 (96 %), no gaps], Mycosphaerella laricis-leptolepidis [strain MAFF 410081, GenBank JX901548.1; Identities = 255/263 (97 %), one gap (0 %)], and Zasmidium corymbiae [strain CBS 145049, GenBank MK047526.1; Identities = 281/306 (92 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Mycodiella sumatrensis [strain CBS 118501, GenBank MF951525.1; Identities = 770/771 (99 %), no gaps], Mycodiella eucalypti [strain CPC 29458, GenBank KY173586.1; Identities = 746/753 (99 %), no gaps], and Collapsimycopappus styracis [strain YS 4, GenBank LC333044.1; Identities = 703/767 (92 %), no gaps]. Closest hits using the tub2 sequence had highest similarity to Mycodiella sumatrensis [strain CBS 118499, GenBank KF902814.1; Identities = 318/323 (98 %), no gaps], Toxicocladosporium rubrigenum [strain CBS 124158, GenBank KY706607.1; Identities = 326/395 (83 %), 21 gaps (5 %)], and Pseudocercospora nelumbonicola [strain RK4111, GenBank LC200982.1; Identities = 321/390 (82 %), 32 gaps (8 %)].
Authors: P.W. Crous, J.Z. Groenewald & M.J. Wingfield
Neobarrmaelia Crous, gen. nov. MycoBank MB 844289.
Taxonomic lineage: Sordariomycetes, Xylariales, incertae sedis.
Etymology: Name reflects its morphological similarity to Barrmaelia.
Mycelium consisting of hyaline, smooth-walled, branched, septate hyphae. Conidiophores erect, brown, smooth-walled, subcylindrical, unbranched, straight to slightly flexuous, 0–2-septate. Conidiogenous cells integrated, terminal, brown, smooth-walled, subcylindrical, forming a rachis of denticles, truncate, not thickened nor darkened. Conidia in dry rosettes, guttulate, hyaline, smooth-walled, subcylindrical, falcate, apical part strongly hooked, aseptate, tapering to subobtuse apex and truncate hilum.
Type species: Neobarrmaelia hyphaenes Crous
Neobarrmaelia hyphaenes Crous, sp. nov. MycoBank MB 844290. Fig. 34.
Fig. 34.
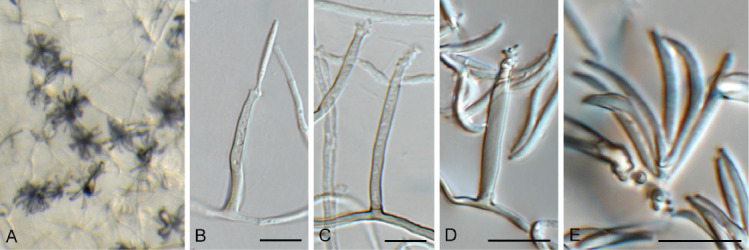
Neobarrmaelia hyphaenes (CPC 40101). A. Conidiophores on SNA. B–D. Conidiophores and conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Hyphaene from which it was isolated.
Mycelium consisting of hyaline, smooth-walled, branched, septate, 1.5–2 μm diam hyphae. Conidiophores erect, brown, smooth-walled, subcylindrical, unbranched, straight to slightly flexuous, 0–2-septate, 25–50 × 3–3.5 μm. Conidiogenous cells integrated, terminal, brown, smooth-walled, subcylindrical, 20–35 × 3–3.5 μm, forming a rachis of denticles, 0.5–1 × 0.5 μm, truncate, not thickened nor darkened. Conidia in dry rosettes, guttulate, hyaline, smooth-walled, subcylindrical, falcate, apical part strongly hooked, aseptate, tapering to subobtuse apex and truncate hilum, 0.5 μm diam, (15–)17–18(–20) × 1.5–2 μm.
Culture characteristics: Colonies erumpent, spreading, surface folded, with moderate aerial mycelium and smooth, lobate margin, reaching 15 mm diam after 2 wk at 25 °C. On MEA surface dirty white, reverse umber with diffuse umber pigment; on PDA surface and reverse dirty white with patches of umber; on OA surface dirty white with patches of isabelline.
Typus: South Africa, KwaZulu-Natal Province, Manguzi, on leaves of Hyphaene sp. (Arecaceae), 19 Oct. 2017, M.J. Wingfield & J. Roux, HPC 3501 (holotype CBS H-24843 culture ex-type CPC 40101 = CBS 148304).
Notes: Neobarrmaelia is a typical xylariaceous asexual morph, resembling the genus Barrmaelia, which is based on B. rhamnicola (Voglmayr et al. 2018, Crous et al. 2020b). Phylogenetically, it groups in a separate clade with B. serenoae, for which the genus Neobarrmaelia is introduced (Fig. 8). Morphologically the two species differ in their conidial dimensions, with those of N. serenoae [(20–)25–30 × 1.5 μm] being longer than conidia of N. hyphaenes [(15–)17–18(–20) × 1.5–2 μm].
Neobarrmaelia serenoae (Crous) Crous, comb. nov. MycoBank MB 844291.
Basionym: Barrmaelia serenoae Crous, Fungal Syst. Evol. 6: 179. 2020.
Material examined: USA, Florida, Gainesville, on leaf of Serenoa repens (Arecaeae), 24 Feb. 2019, M.J. Wingfield, HPC 2792 (holotype CBS H-24201, culture ex-type CPC 37572 = CBS 146017).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Barrmaelia macrospora [strain CBS 142768, GenBank NR_167684.1; Identities = 536/618 (87 %), 18 gaps (2 %)], Barrmaelia moravica [strain CBS 142769, GenBank NR_153495.1; Identities = 536/618 (87 %), 20 gaps (3 %)], and Entosordaria quercina [strain CBS 142774, GenBank NR_153499.1; Identities = 534/619 (86 %), 24 gaps (3 %)]. Closest hits using the LSU sequence are Sarcoxylon compunctum [strain CBS 359.61, GenBank MH869652.1; Identities = 801/821 (98 %), four gaps (0 %)], Xylaria badia [strain 5256, GenBank JQ862643.1; Identities = 798/819 (97 %), no gaps], and Xylaria acuta [strain 5220, GenBank JQ862637.1; Identities = 798/819 (97 %), no gaps]; LSU sequences of Barrmaelia match around 97 %. No significant hits were obtained when the rpb2 and tub2 sequences were used in blastn and megablast searches.
Authors: P.W. Crous, J.Z. Groenewald, J. Roux & M.J. Wingfield
Neobryochiton Crous & Boers, gen. nov. MycoBank MB 844292.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Teratosphaeriaceae.
Etymology: Name reflects its morphological similarity to Bryochiton.
Ascomata pseudothecial, solitary, superficial, globose with central ostiole, brown; wall of 2–3 layers of brown textura angularis. Asci 8-spored, bitunicate with ocular chamber, ellipsoid. Ascospores multiseriate, hyaline, smooth-walled, guttulate, medianly 1-septate, constricted at septum, fusoid-ellipsoid, widest above septum, becoming 3-septate, brown and verruculose with age, surrounded by mucoid sheath.
Type species: Neobryochiton narthecii Crous & Boers
Neobryochiton narthecii Crous & Boers, sp. nov. MycoBank MB 844293. Fig. 35.
Fig. 35.
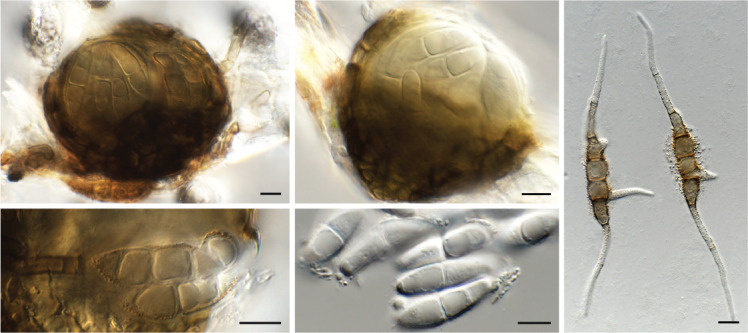
Neobryochiton narthecii (CPC 41972). A, B. Superficial ascomata. C, D. Ascospores. E. Germinating ascospores. Scale bars = 10 μm.
Etymology: Name refers to the genus Narthecium from which it was isolated.
Ascomata pseudothecial, solitary, superficial, globose with central ostiole, brown, 40–70 μm diam; wall of 2–3 layers of brown textura angularis. Asci 8-spored, bitunicate with ocular chamber, ellipsoid, 25–40 × 20–30 μm. Ascospores multiseriate, hyaline, smooth-walled, guttulate (with two large guttules per cell when mounted in water), medianly 1-septate, constricted at septum, fusoid-ellipsoid, widest above septum, becoming 3-septate, brown and verruculose with age, surrounded by mucoid sheath, (22–)25–28(–30) × (7–)8–9 μm. Germinating ascospores with polar germ tubes, germinating parallel to long axis of the spore, later developing secondary lateral germ tubes from middle of ascospore body; ascospores 9–11 μm diam, verruculose, with prominent mucoid sheath.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 7 mm diam after 2 wk. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: Netherlands, Drenthe Province, Dwingelderveld National Park, 52.829188, 6.432495, on dead leaves of Narthecium ossifragum (Nartheciaceae), 4 Jul. 2021, J. Boers, HPC 3653 (holotype CBS H-24993, cultures ex-type CPC 41972, 41973 = CBS 149172).
Notes: Döbbeler (1978) introduced the genus Bryochiton (based on B. monascus; ascomata up to 40 μm diam, with brown, 3-septate, ellipsoid ascospores, 9–12.5 × 3.5–4.5 μm, asci 15–20 × 10–15 μm). Bryochiton presently accommodates five bryosymbiotic species (Döbbeler 2007) with much smaller ascospores, which also differ from N. narthecii in general morphology.
Neobryochiton narthecii is closely related to “Teratosphaeria” encephalarti (Crous et al. 2008), and several isolates identified as “Bryochiton sp.”, in a study that revealed Bryochiton to be paraphyletic (Wäli et al. 2014). Neobryochiton is morphologically distinct from T. encephalarti and Teratosphaeria in general by having 3-septate ascospores as observed in Bryochiton s.str., from which it is phylogenetically distinct (Fig. 1 part 2, 36). Neobryochiton narthecii is easily overlooked as it inhabits decaying leaves of N. ossifragum in peat bogs, intermixed with many other fungi. It can be found by carefully searching for the minute ascomata on leaves from the previous growing season which are lying on top of the Sphagnum layer.
Fig. 36.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 (Minh et al. 2020) of the Teratosphaeriaceae ITS nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Cladosporium halotolerans (culture CBS 119416; GenBank NR_119605.1) and the species treated here are highlighted with coloured blocks and bold face. Accession numbers of sequence from material with a type status are also shown in bold face.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to “Bryochiton” sp. [strain BRD3-083K, GenBank MT133280.1; Identities = 469/480 (98 %), two gaps (0 %)], “Bryochiton” sp. PW-2014 [strain M237, GenBank KM186837.1; Identities = 458/480 (95 %), four gaps (0 %)], and Teratosphaeria encephalarti [strain CPC 15465, GenBank FJ372400.1; Identities = 442/484 (91 %), 13 gaps (2 %)]. Closest hits using the LSU sequence are Bryochiton sp. [strain M198, GenBank EU940130.1; Identities = 793/796 (99 %), no gaps], Teratosphaeria encephalarti [strain CPC 15466, GenBank FJ372418.1; Identities = 786/796 (99 %), no gaps], and Catenulostroma chromoblastomycosum [strain CBS 597.97, GenBank EU019251.2; Identities = 784/796 (98 %), no gaps]. Closest hits using the rpb2 sequence had distant similarity to Constantinomyces oldenburgensis [strain T2.4, GenBank LT976528.1; Identities = 626/799 (78 %), 12 gaps (1 %)], Aulographina pinorum [strain CBS 174.90, GenBank GU371737.1; Identities = 625/817 (76 %), 18 gaps (2 %)], and Teratosphaeria nubilosa [strain CBS 116005, GenBank KT216527.1; Identities = 629/837 (75 %), 24 gaps (2 %)].
Authors: P.W. Crous, J.Z. Groenewald & J. Boers
Neocamarographium Crous, gen. nov. MycoBank MB 844294.
Taxonomic lineage: Dothideomycetes, Pleosporales, incertae sedis.
Etymology: Name reflects its morphological similarity to Camarographium.
Conidiomata pycnidial, numerous, separate, dispersed, single, subepidermal, unilocular, completely immersed in the bark of the host, globose, rarely slightly depressed, with central, ostiolum. Conidiomatal wall up to 100 μm thick, composed at the outer layers of thick-walled, dark brown textura angularis, and at the inner layers of thin-walled, subhyaline textura angularis. Paraphyses intermingled among conidiogenous cells in some conidiomata, hyaline, smooth, subcylindrical with obtuse ends, septate, extending above the conidiogenous cells. Conidiogenous cells hyaline, discrete, holoblastic, annellidic, broadly ampulliform or doliiform. Conidia initially subhyaline, but later becoming yellowish brown in pycnidia, extruding in a slimy mass; young, subhyaline conidia have 3–5 transversal distosepta, whereas in mature conidia the compartments between the septa develop bodies (possible endoconidia) that are ellipsoid to subglobose, thick-walled, verruculose, at times guttulate, and get released in clusters of four, in sacks that appear to be the remnants of the conidial compartments. Outer conidial wall smooth, subhyaline, 1 μm thick; conidia oblong- ellipsoidal or slightly clavate, sometimes with light constriction in median point, with scar at the base.
Type species: Neocamarographium carpini (Melnik et al.) Crous
Neocamarographium carpini (Melnik et al.) Crous, comb. nov. MycoBank MB 844295.
Basionym: Camarographium carpini Melnik et al., Persoonia 27: 149. 2011.
Description and illustration: Mel’nik et al. (2011).
Typus: Russia, St. Petersburg, Botanical Garden of the Komarov Botanical Institute, on thin, dried twigs of Carpinus betulus (Betulaceae), 27 Sept. 2010, V. Mel’nik (holotype LE 226162; paratypes LE 261808, LE 261817; isotypes HAL 2424 F, CBS H-20506), cultures ex-isotype CPC 18919, 18918 = CBS 128781.
Material examined: Germany, hornbeam wood (Carpinion), alt. 44 m a.s.l., sandy soil, acid fresh, mesotroph, on attached, corticated, initial, 1.5–4 mm diam twigs of Carpinus betulus (Betulaceae), 19 Jul. 2014, R.K. Schumacher, RKS 367, culture CPC 25067.
Notes: Neocamarographium is distinct from Camarographium in lacking an eustroma, but with well-defined unilocular conidiomata, and macroconidia that appear to develop internal structures that are possible endoconidia (Mel’nik et al. 2011). Phylogenetically, it also forms a distinct lineage (Fig. 2).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Camarographium carpini [strain CBS 128781, GenBank NR_156250.1; Identities = 514/515 (99 %), no gaps], Pleospora iqbalii [strain CBS 362.69, GenBank MH859323.1; Identities = 700/808 (87 %), 55 gaps (6 %)], and Melanomma populicola [culture CPC 27203, GenBank MT223817.1; Identities = 685/828 (83 %), 40 gaps (4 %)].
Authors: P.W. Crous, R.K. Schumacher, J.Z. Groenewald, M. Starink-Willemse & A.L. van Iperen
Neofusicoccum mediterraneum Crous et al., Fungal Planet, no. 19: [2]. 2007. Fig. 37.
Fig. 37.
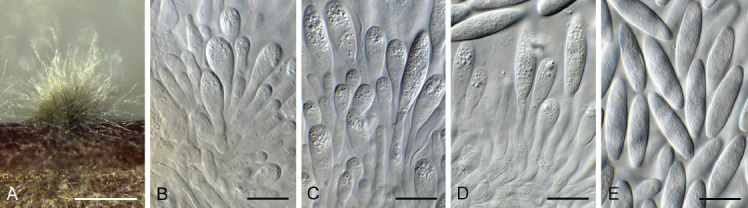
Neofusicoccum mediterraneum (CBS 125263). A. Conidioma on PNA. B–D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars: A = 300 μm, all others = 10 μm.
Taxonomic lineage: Dothideomycetes, Botryosphaeriales, Botryosphaeriaceae.
On PNA: Conidiomata stromatic, pycnidial, immersed, becoming somewhat erumpent, covered with hyphae on PNA, solitary, globose, 200–300 μm diam; wall of 6–8 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells or with a supporting cell at base, hyaline, smooth, subcylindrical, proliferating percurrently at apex, 10–30 × 3–4 μm. Conidia hyaline, smooth, granular, straight, fusoid-ellipsoid, apex subobtuse, base truncate, aseptate, (23–)24–26(–27) × (6–)6.5–7 μm. Dichomera asexual morph not observed.
Culture characteristics: Colonies erumpent, spreading, with fluffy, abundant aerial mycelium and smooth, even margin, covering dish after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Description and illustration: Crous et al. (2007b).
Material examined: South Africa, Gauteng Province, Leeuwfontein Reserve, on Terminalia sericea (Combretaceae), 2010, D. Begoude & J. Roux (CBS H-24558, culture CMW 26679 = CBS 125263).
Notes: Although the name “Neofusicoccum terminaliae” has been used in literature (e.g. Zhang et al. 2021), it is an unpublished taxon from an earlier study on Botryosphaeriaceae occurring on Terminalia in Cameroon, South Africa and Madagascar (Begoude et al. 2010). Morphologically, “N. terminaliae” closely resembles N. mediterraneum (Fig. 37; Crous et al. 2007b) but genetically the introduction of a new taxon for the material from Terminalia is not warranted (Fig. 1 part 1; blast results below) and is herewith assigned to N. mediterraneum. Neofusicoccum mediterraneum was described from branches and leaves of Eucalyptus in Greece and rotting drupes of Olea europaea in Italy (Crous et al. 2007b).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Neofusicoccum mediterraneum [strain ALG77, GenBank KJ657706.1; Identities = 563/565 (99 %), no gaps], Neofusicoccum vitifusiforme [strain CMW 875, GenBank HM176534.1; Identities = 559/565 (99 %), one gap (0 %)], and Neofusicoccum corticosae [now a synonym of Neofusicoccum vitifusiforme; strain CBS 120081, GenBank MN161920.1; Identities = 555/561 (99 %), one gap (0 %)]. Closest hits using the LSU sequence are Neofusicoccum mediterraneum [strain CBS 121718, GenBank NG_069899.1; Identities = 908/908 (100 %), no gaps], Neofusicoccum ningerense [strain CSF6030, GenBank MT029168.1; Identities = 894/896 (99 %), one gap (0 %)], and Neofusicoccum magniconidium [strain CSF5876, GenBank MT029166.1; Identities = 894/896 (99 %), one gap (0 %)]. Closest hits using the rpb2 sequence had highest similarity to Neofusicoccum mediterraneum [strain CBS 121718, GenBank KX464024.1; Identities = 592/594 (99 %), no gaps], Botryosphaeria dothidea [strain B71, GenBank AY217047.1; Identities = 592/594 (99 %), no gaps], and Neofusicoccum ursorum [strain CBS 122811, GenBank KX464047.1; Identities = 589/594 (99 %), no gaps]. Closest hits using the tef1 sequence had highest similarity to Neofusicoccum sp. [strain BJFU B6209, GenBank MH459051.1; Identities = 263/265 (99 %), no gaps], Neofusicoccum ursorum [strain CBS 122812, GenBank KX464760.1; Identities = 262/264 (99 %), no gaps], and Neofusicoccum mediterraneum [strain MAN21312, GenBank KU997247.1; Identities = 272/275 (99 %), no gaps]. Closest hits using the tub2 sequence had highest similarity to Neofusicoccum mediterraneum [strain ColPat-454, GenBank MN839590.1; Identities = 434/434 (100 %), no gaps], Neofusicoccum pistaciarum [now a synonym of Neofusicoccum mediterraneum; strain CBS 113083, GenBank KX464998.1; Identities = 433/434 (99 %), no gaps], and Neofusicoccum ursorum [strain CBS 122811, GenBank KX465056.1; Identities = 432/434 (99 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald, J. Roux & D. Begoude
Niesslia neoexosporioides Crous & R.K. Schumach., Fungal Syst. Evol. 7: 302. 2021. Fig. 38.
Fig. 38.
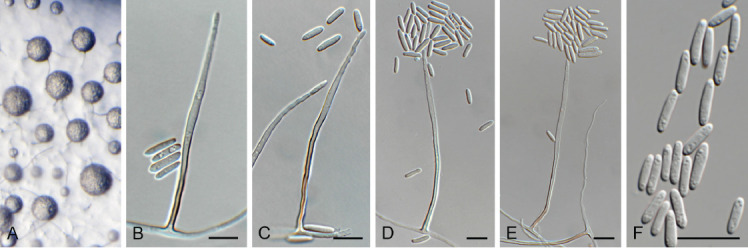
Niesslia neoexosporioides (CPC 41317). A. Conidiophores on SNA. B. Stipe with vesicle. C–E. Conidiophores with conidia. F. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Sordariomycetes, Hypocreales, Niessliaceae.
Description and illustration: Crous et al. (2021e).
Material examined: Netherlands, Utrecht Province, Nieuw Wulven, near Houten, 52°03’03’’N, 05°09’46’’E, 1.5 m a.s.l., on dead culms of Phragmites australis (Poaceae), 25 Feb. 2021, E.R. Osieck, HPC 3613 = WI-32/#4221, culture CPC 41317 = CBS 148284.
Notes: Niesslia neoexosporioides was described from dead leaves of Carex paniculata collected in Germany (Crous et al. 2021e), and the present collection of the asexual morph on dead culms of Phragmites australis from the Netherlands represents a new host record for the species with which it also clusters in the phylogenetic trees (Fig. 7, 39).
Fig. 39.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 (Minh et al. 2020) of the Niesslia ITS nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Trichoderma atroviride (culture CBS 142.95; GenBank MH862505.1) and the species treated here are highlighted with coloured blocks and bold face. Accession numbers of sequence from material with a type status are also shown in bold face.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Niesslia neoexosporioides [strain CBS 146810, GenBank NR_173014.1; Identities = 366/366 (100 %), no gaps], Niesslia aemula [strain CBS 556.75, GenBank MG827004.1; Identities = 362/367 (99 %), one gap (0 %)], and Monocillium curvisetosum [strain CBS 660.94, GenBank NR_160195.1; Identities = 360/365 (99 %), one gap (0 %)]. Closest hits using the LSU sequence are Niesslia neoexosporioides [strain CBS 146810, GenBank NG_076714.1; Identities = 799/799 (100 %), no gaps], Niesslia exosporioides [strain CBS 691.71, GenBank MG826829.1; Identities = 797/799 (99 %), no gaps], and Monocillium curvisetosum [strain CBS 660.94, GenBank MH874141.1; Identities = 795/799 (99 %), no gaps]. The best hit using the actA sequence had highest similarity to Niesslia neoexosporioides [strain CPC 38177, GenBank MW890027.1; Identities = 669/676 (99 %), four gaps (0 %)]; all other hits were too distant. The best hit using the tef1 (first part) sequence had highest similarity to Niesslia neoexosporioides [strain CPC 38177, GenBank MW890097.1; Identities = 475/494 (96 %)]; all other hits were too distant. The best hit using the tub2 sequence had highest similarity to Niesslia neoexosporioides [strain CPC 38177, GenBank MW890137.1; Identities = 642/660 (97 %), seven gaps (1 %)]; all other hits were too distant.
Authors: P.W. Crous, J.Z. Groenewald & E.R. Osieck
Niesslia pseudoexilis Crous & R.K. Schumach., sp. nov. MycoBank MB 844297. Fig. 40.
Fig. 40.
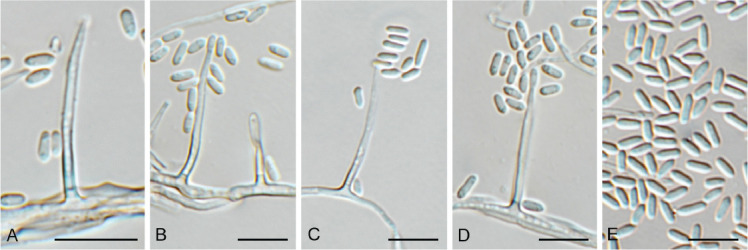
Niesslia pseudoexilis (CPC 40376). A–D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Sordariomycetes, Hypocreales, Niessliaceae.
Etymology: Name refers to the fact that it is closely related to Niesslia exilis.
In OA (sterile on SNA). Mycelium consisting of hyaline, smooth-walled, branched, septate, 1.5–2 μm diam hyphae, forming hyphal coils, and aggregating into hyphal strands that give rise to conidiophores. Conidiophores reduced to conidiogenous cells, hyaline, smooth- and thick-walled, flexuous, 15–30 × 1.5–2 μm, monophialidic, apex 1 μm diam with minute flared collarette that gives rise to a mucoid conidial mass. Conidia solitary, aseptate, hyaline, smooth-walled, guttulate, subcylindrical, apex obtuse, tapering to a truncate hilum, 0.5 μm diam, (3.5–)4–5(–6) × 1.5(–2) μm.
Culture characteristics: Colonies flat, spreading, surface folded, with sparse aerial mycelium and smooth, lobate margin, reaching 10 mm diam after 2 wk at 25 °C. On MEA surface and reverse pale luteous; on PDA and OA surface and reverse dirty white.
Typus: Serbia, Fruška Gora, 45.144876, 19.776186, on dead fallen leaf of Quercus petraea (Fagaceae), 6 Nov. 2020, D. Savić HPC 3543, RKS 1155 (holotype CBS H-24866, culture ex-type CPC 40376 = CBS 148333).
Notes: Niesslia pseudoexilis is phylogenetically closely related to N. exilis (conidia 0–1-septate, rarely multi-septate, 5–11 × 1.2–2 μm; Gams et al. 2019) based on the LSU and ITS phylogenies (Fig. 7, 39), but has smaller conidia. Although a sexual morph was present on the type specimen, the material proved inadequate to facilitate a morphological description.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Niesslia exilis [strain CBS 389.70B, GenBank MG826980.1; Identities = 477/533 (89 %), 13 gaps (2 %)], Myrtacremonium eucalypti [strain CBS 142161, GenBank NR_154212.1; Identities = 470/529 (89 %), 26 gaps (4 %)], and Thyronectria rhodochlora [strain NP8, GenBank KM225682.1; Identities = 476/538 (88 %), 19 gaps (3 %)]. Closest hits using the LSU sequence are Niesslia exilis [strain CBS 389.70B, GenBank MG826782.1; Identities = 788/799 (99 %), one gap (0 %)], Niesslia cladoniicola [strain CBS 960.73, GenBank MG826850.1; Identities = 785/799 (98 %), one gap (0 %)], and Niesslia ilicifolia [strain CBS 459.74, GenBank MG826798.1; Identities = 784/799 (98 %), one gap (0 %)]. Closest hits using the actA sequence had highest similarity to Nectria dacryocarpa [strain CBS 113532, GenBank KM231257.1; Identities = 585/658 (89 %), 27 gaps (4 %)], Dactylonectria alcacerensis [strain CBS 129087, GenBank KM231158.1; Identities = 557/645 (86 %), 32 gaps (4 %)], and Dactylonectria novozelandica [strain CBS 113552, GenBank KM231157.1; Identities = 557/645 (86 %), 32 gaps (4 %)]. Closest hits using the rpb2 sequence had highest similarity to Purpureocillium lilacinum [strain BUP614, GenBank MH879624.1; Identities = 691/833 (83 %), six gaps (0 %)], Isaria takamizusanensis [strain NHJ 3497, GenBank EU369074.1; Identities = 675/817 (83 %), six gaps (0 %)], and Tolypocladium paradoxum [strain NBRC 106958, GenBank AB968561.1; Identities = 691/840 (82 %), two gaps (0 %)]. No significant hits were obtained when the tub2 sequence was used in blastn and megablast searches.
Authors: P.W. Crous, J.Z. Groenewald & R.K. Schumacher
Nothocladosporium Crous, gen. nov. MycoBank MB 844298.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Phillipsiellaceae.
Etymology: Name refers to its morphological similarity to Cladosporium.
Mycelium consisting of hyaline, smooth-walled, branched, septate hyphae. Conidiophores macronematous, solitary, erect, subcylindrical, straight, unbranched, base bulbous, lacking rhizoids; stipe dark brown, smooth- and thick-walled, multiseptate, bearing an apical penicillate arrangement of branches that give rise to dry, branched conidial chains. Penicillate apparatus of primary and secondary branches, giving rise to conidiogenous cells; primary branches brown, aseptate, subcylindrical; secondary branches brown, aseptate, subcylindrical. Conidiogenous cells pale brown, smooth-walled, subcylindrical to clavate; polyblastic, with loci darkened and thickened, not refractive. Conidia occurring in branched chains, aseptate, pale brown, smooth-walled, guttulate; ramoconidia subcylindrical to narrowly fusoid-ellipsoid; conidia fusoid-ellipsoid, hila thickened, darkened, not refractive.
Type species: Nothocladosporium syzygii Crous
Nothocladosporium syzygii Crous, sp. nov. MycoBank MB 844299. Fig. 41.
Fig. 41.
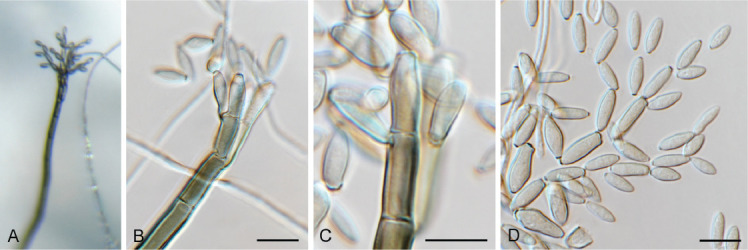
Nothocladosporium syzygii (CPC 40091). A. Conidiophore on SNA. B, C. Conidiophore branches and conidiogenous cells. D. Conidia in chains. Scale bars = 10 μm.
Etymology: Name refers to the host genus Syzygium from which it was isolated.
Mycelium consisting of hyaline, smooth-walled, branched, septate, 1.5–2 μm diam hyphae. Conidiophores macronematous, solitary, erect, subcylindrical, straight, unbranched, base bulbous (10–12 μm diam), lacking rhizoids; stipe dark brown, smooth- and thick-walled, multiseptate, bearing an apical penicillate arrangement of branches that give rise to dry, branched conidial chains. Penicillate apparatus of primary and secondary branches, giving rise to 1–4 conidiogenous cells; primary branches brown, aseptate, subcylindrical, 15–20 × 5–6 μm; secondary branches brown, aseptate, subcylindrical, 8–12 × 4–5 μm. Conidiogenous cells pale brown, smooth-walled, subcylindrical to clavate, 10–16 × 3.5–6 μm; polyblastic, with loci darkened and thickened, not refractive, 2 μm diam. Conidia occurring in branched chains, aseptate, pale brown, smooth-walled, guttulate; ramoconidia subcylindrical to narrowly fusoid-ellipsoid, 10–13 × 4–5 μm; conidia fusoid-ellipsoid, (8–)9–10(–11) × (3–)3.5(–4) μm; hila thickened, darkened, not refractive, 1–1.5 μm diam.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 15 mm diam after 2 wk at 25 °C. On MEA surface pale luteous with patches of ochreous, reverse umber; on PDA surface and reverse pale luteous; on OA surface pale luteous.
Typus: South Africa, near Mozambique, on leaves of Syzygium cordatum (Myrtaceae), 19 Oct. 2017, M.J. Wingfield, HPC 3503 (holotype CBS H-24835, culture ex-type CPC 40091 = CBS 148289).
Notes: Penidiella (Teratosphaeriaceae, Mycosphaerellales), accommodates hyphomycetes with brown, macronematous conidiophores with a conidiogenous apparatus composed of several branches, giving rise to ramoconidia and conidia with hila that are unthickened or almost so, barely to somewhat darkened-refractive (Crous et al. 2007a). Cladosporium s.str. (Cladosporiaceae, Cladosporiales) is distinguished by having conidia with typical coronate conidiogenous loci and conidial hila, i.e., with a convex central dome surrounded by a raised periclinal rim (Bensch et al. 2012). In Nothocladosporium the conidiophores appear cladosporium-like, but the conidiogenous loci and conidial hila resemble those of Penidiella. Phylogenetically, however, it clusters apart, being more closely related to ascomycetes such as Johansonia and Schizothyrium (Crous et al. 2010), and therefore a new genus is introduced here to accommodate it (Fig. 1 part 2, 13 part 1).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Johansonia chapadensis [strain CBS 128068, GenBank HQ423449.1; Identities = 386/452 (85 %), 31 gaps (6 %)], Schizothyrium pomi [strain SP12_375Aa, GenBank MN065457.1; Identities = 451/517 (87 %), 18 gaps (3 %)], Zygophiala trispora [strain HMAS 244987, GenBank NR_159004.1; Identities = 451/516 (87 %), 21 gaps (4 %)], and Zygophiala trispora [as Zygophiala sp. GS-2014b; strain HL-HKBJ-23D, GenBank KF646711.1; Identities = 451/516 (87 %), 21 gaps (4 %)]. Closest hits using the LSU sequence are Johansonia chapadensis [strain CBS 128068, GenBank NG_057893.1; Identities = 785/798 (98 %), no gaps], Schizothyrium pomi [strain SP12_375Aa, GenBank MN065457.1; Identities = 784/799 (98 %), one gap (0 %)], and Schizothyrium cylindricum [strain SP12_377Ha, GenBank MN065458.1; Identities = 783/799 (98 %), one gap (0 %)].Closest hits using the actA sequence had highest similarity to Pseudocercosporella myopori [strain CBS 114644, GenBank JX143245.2; Identities = 367/399 (92 %), no gaps], Pseudocercospora neriicola [strain CBS 138010, GenBank KJ869231.1; Identities = 367/399 (92 %), no gaps], and Pseudocercospora paraguayensis [strain CBS 111317, GenBank JQ325021.1; Identities = 367/399 (92 %), no gaps]. Closest hits using the tef1 (first part) sequence had highest similarity to Neotrimmatostroma paraexcentricum [strain CPC 25594, GenBank KX228378.1; Identities = 345/422 (82 %), 27 gaps (6 %)], Neotrimmatostroma dalrympleanae [strain CBS 144609, GenBank MN162339.1; Identities = 327/397 (82 %), 22 gaps (5 %)], and Phaeothecoidea intermedia [strain CBS 124994, GenBank KF903171.1; Identities = 302/372 (81 %), 21 gaps (5 %)]. No significant hits were obtained when the tub2 sequence was used in blastn and megablast searches.
Authors: P.W. Crous, J.Z. Groenewald & M.J. Wingfield
Nothopseudocercospora Crous & U. Braun, gen. nov. MycoBank MB 844415.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Mycosphaerellaceae.
Etymology: Resembling the genus Pseudocercospora.
Phytopathogenic. Leaf spots amphigenous, angular to irregular, pale brown with dark brown border. Conidiomata with well-developed stroma, erumpent, brown, consisting of pale brown pseudoparenchymatal cells. Conidiophores subcylindrical, pale brown, smooth-walled, unbranched, septate. Conidiogenous cells terminal, integrated, subcylindrical, pale brown, smooth-walled, holoblastic, at times sympodial, or with minute percurrent proliferation. Conidia solitary, subcylindrical, straight to gently curved, apices subobtuse, base truncate, not thickened nor darkened, pale olivaceous, guttulate, septate, with flared basal marginal frill.
Type species: Nothopseudocercospora dictamni (Fuckel) Crous & U. Braun
Nothopseudocercospora dictamni (Fuckel) Crous & U. Braun, comb. nov. MycoBank MB 844279. Fig. 42.
Fig. 42.
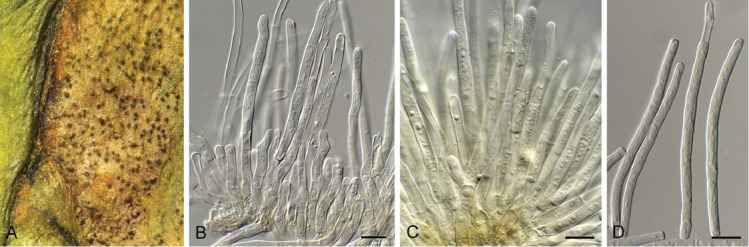
Nothopseudocercospora dictamni (CPC 39776). A. Leaf spot with conidiophores fascicles. B, C. Conidiogenous cells giving rise to conidia. D. Conidia. Scale bars = 10 μm.
Basionym: Septoria dictamni Fuckel, Enumeratio Fungorum Nassoviae: 330. 1860 (Jahrb. Vereins Naturk. Herzogth. Nassau: 15. 1860).
Synonyms: Cercospora dictamni (Fuckel) Vassiljevsky, in Vassiljevsky & Karakulin, Parazitnye nesovershennye griby, 2, Melankonialnye: 574, Leningrad 1950.
Cercostigmina dictamni (Fuckel) U. Braun, Cryptogam. Bot. 4: 108. 1993.
Pseudocercospora dictamni (Fuckel) U. Braun & Crous, Mycol. Prog. 1: 22. 2002.
Leaf spots amphigenous, angular to irregular, 5–12 mm diam, pale brown with dark brown border. Conidiomata mainly epiphyllous, stroma well-developed, erumpent, brown, consisting of pale brown pseudoparenchymatal cells, up to 120 × 70 μm. Conidiophores subcylindrical, pale brown, smooth-walled, unbranched, 1–2-septate, 20–40 × 4–6 μm. Conidiogenous cells terminal, integrated, subcylindrical, pale brown, smooth-walled, holoblastic, at times sympodial, or with minute percurrent proliferation, 15–25 × 4–6 μm. Conidia solitary, subcylindrical, straight to gently curved, apices subobtuse, base truncate, not thickened nor darkened, pale olivaceous, guttulate, (1–)3–4(–7)-septate, (50–)55–85(–120) × (3.5–)4(–5) μm, with flared basal marginal frill.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 20 mm diam after 7 d at 25 °C. On MEA surface pale white, reverse red; on PDA surface pale white with patches of olivaceous, reverse red with diffuse red pigment; on OA surface pale white with diffuse red pigment.
Material examined: Germany, on Dictamnus albus (Rutaceae), Fungi Rhen. 519 lectotype (HAL), designated in Braun, Crypt. Bot 3: 243. 1993. Russia, Rostov region, Krasnosulinsky district, state natural wildlife area “Gornensky”, stony steppe on a slope of a gully, on living leaves of Dictamnus albus, 27 Jun. 2020, T.S. Bulgakov, HPC 3399 = PC 062 (epitype designated here MBT 10007397, CBS H-24838, isoepitype LE F-332410, culture ex-epitype CPC 39776 = CBS 148299).
Notes: Nothopseudocercospora dictamni was originally described as a foliar pathogen of Dictamnus albus collected in Germany. The present collection matches the morphology of the lectotype (Braun 1993, Braun & Hill 2002), except for having slightly wider conidiophores, and is thus an appropriate candidate for epitypification. Although it fits the genus Pseudocercospora based on gross morphology, it also resembles Collarispora, which is characterised by subcylindrical conidia that are initially hyaline but later become pale brown, with a basal marginal frill. Nothopseudocercospora is closer related to Sultanimyces (conidiogenous cells polyblastic; conidiogenous loci conspicuous and slightly protruding; conidia pale to moderate olivaceous, ellipsoid, fusoid, subcylindrical or obclavate, smooth to verruculose, usually septate, median septum usually thicker, sometimes slightly constricted at median septum, with conspicuous and slightly protruding hila) and Neocercosporidium (conidiogenous cells integrated, terminal, proliferating sympodially, occasionally percurrently, conidiogenous loci minute but slightly thickened, darkened and refractive, front view resembling minute circles; conidia subhyaline to pale olivaceous or brownish, smooth, thin-walled, multi-septate, obclavate-cylindrical; hila slightly thickened, darkened and refractive) (Videira et al. 2017), but does not fit in either, and is thus introduced here as a new genus (Fig. 1 part 2, 13 part 2).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Amycosphaerella africana [strain CBS 144635, GenBank MK442569.1; Identities = 495/509 (97 %), no gaps], Clarohilum henningsii [strain IR-9, GenBank LC565143.1; Identities = 494/509 (97 %), no gaps], and Cercosporidium californicum [strain CBS 128857, GenBank MH865121.1; Identities = 494/510 (97 %), one gap (0 %)]. Closest hits using the LSU sequence are Passalora vicosae [strain URM 8138, GenBank MN707422.1; Identities = 805/808 (99 %), no gaps], Pseudocercosporella bidentis [strain CPC 19493, GenBank NG_069168.1; Identities = 804/808 (99 %), no gaps], and Collarispora valgourgensis [strain CBS 129531, GenBank MH878078.1; Identities = 804/808 (99 %), no gaps]. Closest hits using the actA sequence had highest similarity to Amycosphaerella africana [as Mycosphaerella ellipsoidea; strain CBS 110843, GenBank JX902106.1; Identities = 489/527 (93 %), six gaps (1 %)], Rhachisphaerella mozambica [as Mycosphaerella mozambica; strain CBS 121391, GenBank EU514319.1; Identities = 483/522 (93 %), five gaps (0 %)], and Pantospora guazumae [strain CIRAD-AUS 130, GenBank MW070787.1; Identities = 528/571 (92 %), five gaps (0 %)]. Closest hits using the cmdA sequence had highest similarity to Nothopassalora personata [strain IRAN 3479C, GenBank MN422408.1; Identities = 396/433 (91 %), five gaps (1 %)], Dothistroma pini [strain CBS 121011, GenBank KF253965.1; Identities = 369/406 (91 %), nine gaps (2 %)], and Dothistroma septosporum [strain CBS 383.74, GenBank KF253966.1; Identities = 380/435 (87 %), 12 gaps (2 %)]. Closest hits using the rpb2 sequence had highest similarity to Cercosporidium californicum [strain CPC 18390, GenBank MF951471.1; Identities = 793/857 (93 %), no gaps], Paracercosporidium tiliae [strain CBS 112734, GenBank MF951565.1; Identities = 793/863 (92 %), no gaps], and Collarispora valgourgensis [strain CBS 125311, GenBank MF951480.1; Identities = 611/665 (92 %), no gaps]. Closest hits using the tef1 (first part) sequence had highest similarity to Paracercospora egenula [strain CBS 485.81, GenBank GU384415.2; Identities = 289/340 (85 %), 22 gaps (6 %)], Amycosphaerella africana [strain CBS 144635, GenBank MK442688.1; Identities = 297/357 (83 %), 39 gaps (10 %)], and Ragnhildiana diffusa [strain IL-GG-1, GenBank MG836579.1; Identities = 290/349 (83 %), 29 gaps (8 %)].
Authors: P.W. Crous, J.Z. Groenewald & U. Braun
Nothotrimmatostroma corymbiae Crous, sp. nov. MycoBank MB 844300. Fig. 43.
Fig. 43.
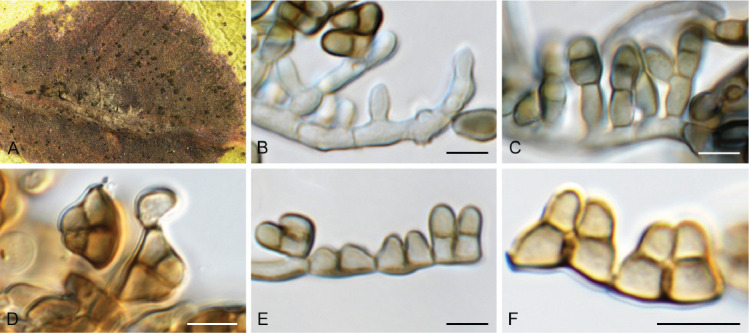
Nothotrimmatostroma corymbiae (CPC 40087). A. Leaf spot. B–E. Conidiogenous cells giving rise to chains of conidia. F. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Teratosphaeriaceae.
Etymology: Name refers to the host genus Corymbia from which it was isolated.
Leaf spots corky, dark brown, irregular to subcircular, 5–12 mm diam, not extending through leaf lamina, margin irregular. Sporodochia dark brown, in concentric clusters, 150–200 μm diam. Conidiophores micronematous, branched, septate, medium brown, smooth-walled, densely aggregated, up to 60 μm tall, 2–3 μm wide. Conidiogenous cells holothallic, integrated, terminal and lateral, doliiform to subcylindrical, pale brown, smooth-walled, 5–10 × 2.5–3.5 μm. Conidia in branched chains, smooth-walled, medium brown, 4-celled, upper two primary cells 6–8 × 6–8 μm, with truncate ends where attached, 2–3 μm diam, separated from each other by a broad brown area, each primary cell giving rise to a smaller basal cell, globose, thin-walled, pale brown, 6–7 × 2.5–4 μm.
Culture characteristics: Colonies erumpent, spreading, with sparse to moderate aerial mycelium and smooth, lobate margin, reaching 6 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: South Africa, KwaZulu-Natal Province, Manguzi, on leaves of Corymbia henryi (Myrtaceae), 19 Oct. 2015, M.J. Wingfield & J. Roux, HPC 3504 (holotype CBS H-24867, culture ex-type CPC 40087 = CBS 148334).
Additional materials examined: South Africa, near Mozambique, on C. henryi, 19 Oct. 2015, M.J. Wingfield, HPC 3502, culture CPC 40085 = CBS 148335; near Mozambique, on C. henryi, 19 Oct. 2015, M.J. Wingfield, HPC 3496, culture CPC 40077 = CBS 148336.
Notes: Nothotrimmatostroma spp. are generally regarded as foliar pathogens of minor importance of Eucalyptus spp. Nothotrimmatostroma corymbiae is closely related to N. bifarium (conidia 12–24 × 6–14 μm, 6–10-celled when mature; Crous et al. 2019c) based on the LSU alignment (Fig. 1, part 2), and is the first species of the genus reported from South Africa. Based om the ITS alignment (Fig. 44), the closest phylogenetic neighbour is Nothotrimmatostroma eucalyptorum, although the connecting node is not supported.
Fig. 44.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 (Minh et al. 2020) of the Nothotrimmatostroma ITS nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Cladosporium cladosporioides (culture CBS 112388; GenBank NR_119839.1) and the species treated here is highlighted with bold face. Accession numbers of sequence from material with a type status are also shown in bold face.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 40087 had highest similarity to Nothotrimmatostroma bifarium [strain CBS 143488, GenBank NR_165605.1; Identities = 496/507 (98 %), no gaps], Nothotrimmatostroma eucalyptorum [strain CBS 129578, GenBank NR_159795.1; Identities = 497/512 (97 %), one gap (0 %)], and Phaeothecoidea intermedia [strain CBS 124994, GenBank MH863442.1; Identities = 495/511 (97 %), no gaps]. The ITS sequence of CPC 40087 is identical to those of CPC 40077 and 40085 (506/506 and 501/501 nucleotides, respectively). Closest hits using the LSU sequence of CPC 40087 are Nothotrimmatostroma bifarium [strain CBS 143488, GenBank NG_067914.1; Identities = 813/814 (99 %), no gaps], Nothotrimmatostroma eucalyptorum [strain CBS 129578, GenBank NG_064248.1; Identities = 816/818 (99 %), no gaps], and Phaeothecoidea intermedia [strain CBS 124994, GenBank NG_057841.1; Identities = 866/871 (99 %), no gaps]. The LSU sequences of CPC 40077, 40085 and 40087 are identical.
Authors: P.W. Crous, J.Z. Groenewald, J. Roux & M.J. Wingfield
Paracamarographium Crous, gen. nov. MycoBank MB 844301.
Taxonomic lineage: Dothideomycetes, Pleosporales, Didymosphaeriaceae.
Etymology: Name reflects its morphological similarity to Camarographium.
Conidiomata pycnidial, subperidermal, separate, single or in small clusters but without connecting stroma, completely immersed in the bark of the host, globose or slightly depressed, with a central, ostiolum; conidiomatal wall composed of a thick outer layer of textura angularis with dark brown cells, and a thick inner layer of textura angularis-globulosa of yellowish to hyaline, thin‐walled cells, giving rise to conidiogenous cells all over the inner wall surface. Macroconidiogenous cells hyaline, discrete, holoblastic, annellidic, broadly ampulliform to doliiform. Macroconidia initially hyaline, elongated‐ellipsoidal to cylindrical, broadly rounded at the apex, rounded and with a scar at the base, 1-celled and thin-walled and containing numerous small oil-droplets when seceded, conidia attaining an up to 1 μm thickened outer wall, and becoming medianly 1-euseptate, then transversely 3-septate, and finally multi-celled because of the formation of numerous distosepta oriented in all directions; each cell filled with 1 or more relatively large oil-droplets, outer conidium wall slowly becoming pale olivaceous brown. Microconidiogenous cells mostly arising from the upper part of the conidiomatal wall, hyaline, discrete, holoblastic, non-proliferating, ampulliform to doliiform. Microconidia hyaline, subglobose to ellipsoidal, rounded at the apex, broadly truncate at the base.
Type species: Paracamarographium koreanum (Verkley et al.) Crous
Paracamarographium koreanum (Verkley et al.) Crous, comb. nov. MycoBank MB 844302.
Basionym: Camarographium koreanum Verkley et al., Sydowia 57: 260. 2005.
Description and illustration: Verkley et al. (2005).
Typus: Korea, Seoul, Hongneung Arboretum, Korea Forest Research Institute, on dead twigs of Cornus kousa (Cornaceae), V. Mel’nik s. n., 10 Sep. 2004 (holotype LE 226162; isotypes L, CBS H-14254; living ex-type cultures CBS 117158, 117159).
Notes: Paracamarographium is distinct from Neocamaro-graphium and Camarographium by having microconidia, and forming separate, single or aggregated pycnidial conidiomata without connecting stroma as observed in Camarographium. Initial analyses (data not shown) of the ITS sequence of Paracamarographium koreanum (GenBank JQ044432.1) indicated a distant relation to isolates identified as Hendersonia pinicola (conidia brown, fusoid-ellipsoid to clavate, 3-septate, from living needles of Pinus murrayana, USA; Wehmeyer 1946). The latter isolates appear to be incorrectly identified, and therefore no new combination is proposed for H. pinicola. Paracamarographium is related to species in Didymosphaeriaceae (Pleosporales; Fig. 2).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CBS 117159 (GenBank JQ044432.1) had distant similarity to Corynespora smithii [strain L133, GenBank KY984299.1; Identities = 882/1 108 (80 %), 80 gaps (7 %)], Bambusicola thailandica [strain MFLUCC 11-0147, GenBank KU940119.1; Identities = 669/805 (83 %), 41 gaps (5 %)], and Bambusicola subthailandica [voucher SICAU 16-0005, GenBank MK253474.1; Identities = 739/909 (81 %), 49 gaps (5 %)]. Closest hits using the LSU sequence of CBS 117159 (JQ044451.1) are Pseudochaetosphaeronema ginkgonis [strain SYP-F-7195, GenBank KU365985.1; Identities = 912/931 (98 %), 10 gaps (1 %)], Massaria platani [strain AFTOL-ID 1574 = CBS 221.37, GenBank DQ678065.1; Identities = 897/917 (98 %), four gaps (0 %)], and Macrodiplodiopsis desmazieri [strain L138, GenBank KR873274.1; Identities = 872/893 (98 %), four gaps (0 %)].
Authors: P.W. Crous, J.Z. Groenewald, M. Starink-Willemse & A.L. van Iperen
Paraphoma salicis Crous & Akulov, Fungal Syst. Evol. 7: 312. 2021. Fig. 45.
Fig. 45.
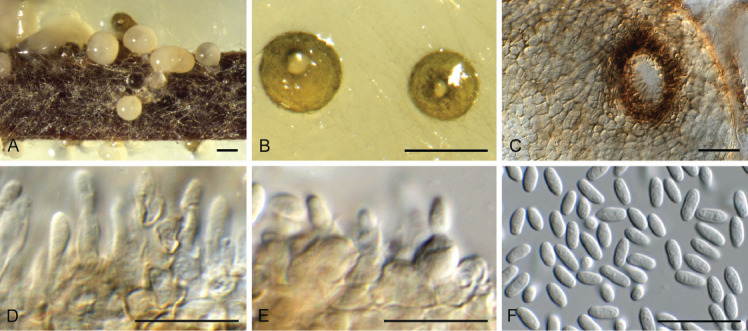
Paraphoma salicis (CPC 39991). A. Conidiomata on PNA. B. Conidiomata on SNA. C. Ostiole. D, E. Conidiogenous cells giving rise to conidia. F. Conidia. Scale bars: A, B = 250 μm, C = 40 μm, all others = 10 μm.
Taxonomic lineage: Dothideomycetes, Pleosporales, Phaeosphaeriaceae.
Conidiomata solitary, erumpent, globose, brown, 150–250 μm diam, with central, dark brown ostiole, 30–40 μm diam. Conidiophores reduced to conidiogenous cells lining inner cavity, hyaline, smooth, ampulliform, 4–7 × 4–5 μm, proliferating percurrently at apex. Conidia aseptate, solitary, hyaline, smooth, finely guttulate, straight, apex obtuse, base bluntly rounded to truncate, (5–)5.5–6(–7) × 2(–2.5) μm.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 30 mm diam after 7 d at 25 °C. On MEA surface ochreous, reverse isabelline; on PDA surface and reverse isabelline; on OA surface isabelline.
Material examined: Netherlands, Gelderland Province, Beuningen, river Waal, on Salix sp. (Salicaceae), 17 Oct. 2020, A.L. van Iperen, HPC 3485 = CBS H-24896, culture CPC 39991 = CBS 148454.
Notes: Paraphoma salicis [conidia (4–)5(–6) × (2–)2.5(–3) μm] was described on leaves of Salix cf. alba collected from Ukraine (Crous et al. 2021b), and is newly recorded here on a Salix sp. from the Netherlands (Fig. 2).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Paraphoma salicis [strain CBS 146797, GenBank NR_173017.1; Identities = 572/573 (99 %), no gaps], Paraphoma radicina [strain LYZ187I187, GenBank MH429796.1; Identities = 440/483 (91 %), 10 gaps (2 %)], and Paraphoma chrysanthemicola [strain MX, GenBank MK426809.1; Identities = 494/550 (90 %), 20 gaps (3 %)]. Closest hits using the LSU sequence are Paraphoma chrysanthemicola [strain AJP07, GenBank KP690077.1; Identities = 839/842 (99 %), no gaps], Paraphoma dioscoreae [strain CBS 135100, GenBank KF251671.1; Identities = 827/830 (99 %), no gaps], and Paraphoma melnikiae [voucher MF-9.182.1, GenBank MG764068.1; Identities = 838/842 (99 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Paraphoma salicis [strain CPC 38651, GenBank MW890069.1; Identities = 876/876 (100 %), no gaps], Paraphoma melnikiae [voucher MF-9.95, GenBank MG779462.1; Identities = 668/735 (91 %), no gaps], and Paraphoma fimeti [strain UTHSC DI16-296, GenBank LT797032.1; Identities = 762/877 (87 %), four gaps (0 %)]. Closest hits using the tub2 sequence had highest similarity to Paraphoma salicis [strain CPC 38651, GenBank MW890140.1; Identities = 440/443 (99 %), no gaps], Paraphoma chrysanthemicola [strain CBS 172.70, GenBank KF252660.1; Identities = 217/230 (94 %), two gaps (0 %)], and Paraphoma melnikiae [voucher MF-9.240, GenBank MG779453.1; Identities = 398/428 (93 %), two gaps (0 %)].
Authors: P.W. Crous, J.Z. Groenewald, M. Starink-Willemse & A.L. van Iperen
Periconia pseudobyssoides S. Markovskaja & A. Kačergius, Mycol. Prog. 13: 293. 2014. Fig. 46.
Fig. 46.
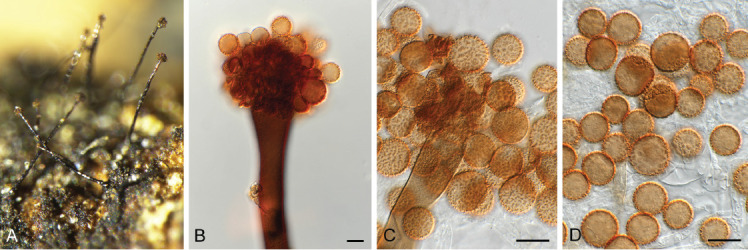
Periconia pseudobyssoides (CPC 38820). A. Conidiophores. B. Conidiophore and conidiogenous cells giving rise to conidia. C. Conidiogenous cells and conidia. E. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Dothideomycetes, Pleosporales, Periconiaceae.
Mycelium consisting of brown, smooth, branched, septate, 3–5 μm diam hyphae. Conidiophores 450–650 × 17–27 μm, solitary, or in aggregated clusters on host tissue, brown, arising from a brown stroma, with base slightly swollen, 30–50 μm diam, with rhizoids; stalk cylindrical, straight, flexuous, thick-walled, smooth, but verruculose in upper cell, 3–4-septate, with large guttules. Primary branches doliiform, brown, verruculose, aseptate, 8–12 × 7–9 μm. Conidiogenous cells terminal and intercalary, or directly on conidiophore, 10–12 × 9–11 μm, tretic. Conidia aseptate, spherical, brown, with delicate spines, in short chains, (11–)13–16(–18) μm diam.
Culture characteristics: Colonies flat, spreading, with sparse to moderate aerial mycelium and smooth, even margin, covering dish after 2 wk at 25 °C. On MEA, PDA and OA surface umber with patches of dirty white, reverse umber with patches of chestnut.
Material examined: South Africa, Northern Province, Nelspruit, Lowveld Botanical Garden, on leaf litter of Euphorbia ingens (Euphorbiaceae), Nov. 2018, P.W. Crous, HPC 3140 = CBS H-24530, culture CPC 38820 = CBS 147067.
Notes: Phylogenetically (Fig. 2) and morphologically, the present collection is close to P. pseudobyssoides (conidiophores 300–600 μm long, conidiogenous cells 10–12 × 6–7.5 μm, conidia (12–) 15–17(–20) μm; Markovskaja & Kačergius 2014).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Periconia byssoides [strain 7GJ-1, GenBank KR708986.1; Identities = 524/526 (99 %), two gaps (0 %)], Periconia pseudobyssoides [strain DUCC 0850, GenBank MG333491.1; Identities = 495/498 (99 %), no gaps], and Acremoniella rugulosa [strain CBS 110.35, GenBank MH855598.1; Identities = 501/506 (99 %), one gap (0 %)]. Closest hits using the LSU sequence are Periconia pseudobyssoides [strain H 4151, GenBank AB807568.1; Identities = 879/880 (99 %), no gaps], Periconia byssoides [strain C292, GenBank MK347968.1; Identities = 884/889 (99 %), no gaps], and Periconia submersa [strain MFLUCC 16-1098, GenBank KY794706.1; Identities = 825/836 (99 %), no gaps]. No significant hits were obtained when the tef1 (first part) sequence was used in blastn and megablast searches. Closest hits using the tef1 (second part) sequence had highest similarity to Periconia byssoides [strain MFLUCC 17-2292, GenBank MK360069.1; Identities = 366/370 (99 %), no gaps], Periconia pseudobyssoides [strain EF61a, GenBank AB808544.1; Identities = 430/436 (99 %), no gaps], and Periconia homothallica [strain EF58a, GenBank AB808541.1; Identities = 415/436 (95 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald & M.J. Wingfield
Phaeoisaria clematidis (Fuckel) S. Hughes, Canad. J. Bot. 36: 794. 1958. Fig. 47.
Fig. 47.
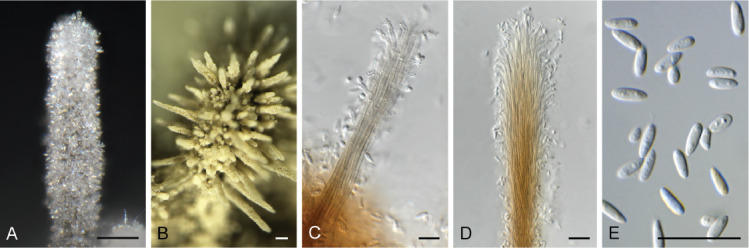
Phaeoisaria clematidis (CPC 39680). A, B. Synnemata. C, D. Synnemata with conidiogenous cells. E. Conidia. Scale bars: A–D = 40 μm, E = 10 μm.
Basionym: Stysanus clematidis Fuckel, Jb. nassau. Ver. Naturk. 23–24: 365. 1870. (1869–1870).
Taxonomic lineage: Sordariomycetes, Pleurotheciales, Pleurotheciaceae.
Synnemata erect, cylindrical to slightly clavate, up to 2 mm long, 20–40 μm diam at base, composed of dark brown stipe of parallel, smooth-walled, septate, branched, 2–3 μm diam hyphae, giving rise to terminal and lateral conidiogenous cells in the upper region of conidiophore. Conidiogenous cells pale brown, smooth, subhyaline towards apex, cylindrical to clavate, 5–15 × 2–2.5 μm; apical rosette of denticles cylindrical, 1–2 × 1 μm, not thickened nor darkened. Conidia aseptate, smooth- and thin-walled, hyaline to subhyaline, subcylindrical or obovoid, straight, (5–)6–7 × 2(–2.5) μm. Chlamydospores produced in agar, thick- and smooth-walled, aseptate, ovoid to subglobose, 10–15 × 8–10 μm.
Culture characteristics: Colonies flat, spreading, with sparse aerial mycelium, surface folded, margin smooth, lobate, reaching up to 8 mm diam after 7 d. On MEA and PDA surface and reverse isabelline; on OA surface honey.
Materials examined: Netherlands, Utrecht Province, De Bilt, Beukenburglaan, on stems of Sambucus nigra (Adoxaceae), 23 Jul. 2020, P.W. Crous, culture CPC 39680; Zeeland Province, Oranjezon, on stems of Sambucus nigra, 17 Sep. 2020, A.L. van Iperen, HPC 3508, CBS H-24994, culture CPC 39937 = CBS 149173.
Notes: The genus Phaeoisaria is easily recognised by its erect synnemata, denticulate conidiogenous cells, and aseptate conidia. Phaeoisaria clematidis, the type species of the genus (Pleurotheciaceae, Pleurotheciales; Fig. 6), appears to be common on dead stems of Sambucus nigra in the Netherlands.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 39680 had highest similarity to Phaeoisaria clematidis [strain GZCC19-0541, GenBank MW133889.1; Identities = 525/530 (99 %), two gaps (0 %)], Hoehneliella perplexa [strain NN057688, GenBank OL627919.1; Identities = 483/489 (99 %), three gaps (0 %)], and Phaeoisaria annesophieae [voucher MFLU 19-0531, GenBank MT559109.1; Identities = 518/549 (94 %), nine gaps (1 %)]. The ITS sequences of CPC 39680 and 39937 are identical (560/560 bp). Closest hits using the LSU sequence of CPC 39680 are Phaeoisaria clematidis [strain MFLUCC 18-1017, GenBank MW132065.1; Identities = 795/799 (99 %), no gaps], Phaeoisaria annesophieae [voucher MFLU 19-0531, GenBank MT559084.1; Identities = 813/822 (99 %), no gaps], and Phaeoisaria filiformis [strain MFLUCC 18-0214, GenBank NG_068642.1; Identities = 774/784 (99 %), no gaps]. The LSU sequences of CPC 39680 and 39937 are identical (808/808 bp). Closest hits using the rpb2 sequence of CPC 39680 had highest similarity to Phaeoisaria clematidis [strain MFLUCC 17-1341, GenBank MF401400.1; Identities = 485/497 (98 %), no gaps], Phaeoisaria aquatica [strain MFLUCC 16-1298, GenBank MF401406.1; Identities = 620/678 (91 %), no gaps], and Phaeoisaria microspora [strain M0104, GenBank MF167352.1; Identities = 668/757 (88 %), seven gaps (0 %)]. The rpb2 sequences of CPC 39680 and 39937 differ at two nucleotides (849/851 bp identical). Closest hits using the SSU sequence of CPC 39680 are Phaeoisaria clematidis [strain MFLUCC 17-1968, GenBank MG837027.1; Identities = 991/991 (100 %), no gaps], Phaeoisaria fasciculata [strain DAOM 230055, GenBank KT278694.1; Identities = 925/928 (99 %), no gaps], and Phaeoisaria guttulata [voucher MFLU 18-0139, GenBank MG837026.1; Identities = 987/991 (99 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald, M. Starink-Willemse & A.L. van Iperen
Phaeophleospora hymenocallidicola Crous, Persoonia 34: 211. 2015. Fig. 48.
Fig. 48.
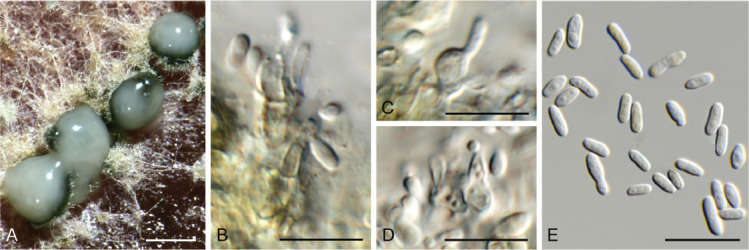
Phaeophleospora hymenocallidicola (CPC 40026). A. Conidiomata on MEA. B–D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars: A = 300 μm, all others = 10 μm.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Mycosphaerellaceae.
Material examined: USA, Puerto Rico, Agricultural experiment station, Juana Diaz, leaf necrosis on Mangifera indica var. Palmer (Anacardiaceae), Jun. 2020, A.M. Quimbita, CBS H-24795, culture CPC 40026 = CBS 148250; Puerto Rico, Commercial farm, Santa Isabel, necrosis on fruit peduncle of M. indica, Jun. 2020, A.M. Quimbita, culture CPC 40027 = CBS 149175.
Notes: Phaeophleospora hymenocallidicola (Mycosphaerellaceae, Mycosphaerellales; Fig. 1 part 2) was originally described as a foliar pathogen of a fern collected in Thailand (Crous et al. 2015), and is linked here to a foliar and fruit peduncle disease of mango in Puerto Rico.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Phaeophleospora hymenocallidicola [strain CBS 139912, GenBank NR_137994.1; Identities = 571/571 (100 %), no gaps], Phaeophleospora eucalypticola [strain CBS 141294, GenBank NR_145123.1; Identities = 570/572 (99 %), no gaps], and Phaeophleospora pteridivora [strain COAD 1182, GenBank NR_155664.1; Identities = 450/481 (94 %), 12 gaps (2 %)]. The ITS sequences of CPC 40026 and 40027 are identical (573/573 bp). Closest hits using the LSU sequence of CPC 40026 are Phaeophleospora eucalypticola [strain CBS 141294, GenBank NG_069357.1; Identities = 813/813 (100 %), no gaps], Phaeophleospora hymenocallidicola [strain CBS 139912, GenBank KR476772.1; Identities = 805/805 (100 %), no gaps], and Phaeophleospora eugeniae [strain CPC 15159, GenBank FJ493207.1; Identities = 810/813 (99 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald, A.M. Quimbita & L.I. Rivera-Vargas
Phaeosphaeria hyphaenes Crous, sp. nov. MycoBank MB 844305. Fig. 49.
Fig. 49.
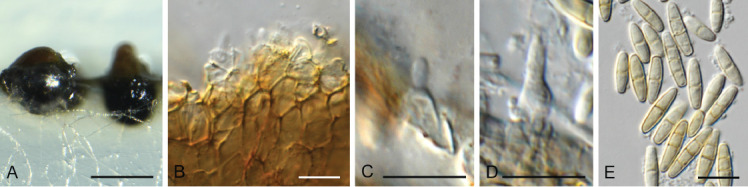
Phaeosphaeria hyphaenes (CPC 40354). A. Conidiomata on SNA. B–D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars: A = 300 μm, all others = 10 μm.
Taxonomic lineage: Dothideomycetes, Pleosporales, Phaeosphaeriaceae.
Etymology: Name refers to the host genus Hyphaene from which it was isolated.
Conidiomata pycnidial, solitary, immersed to erumpent, globose, brown with central ostiole, 150–300 μm diam; wall of 3–5 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity. Conidiogenous cells subhyaline, smooth, ampulliform to doliiform, 4–5 × 3–5 μm; proliferating inconspicuously percurrently at apex. Conidia solitary, pale brown, smooth-walled, guttulate, subcylindrical, straight, apex obtuse, base truncate with flat scar, 0.5 μm diam, 1–3-septate, (7–)9–12(–13) × (2.5–)3 μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 45 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: South Africa, KwaZulu-Natal Province, Manguzi, on leaves of Hyphaene sp. (Arecaceae), 19 Oct. 2017, M.J. Wingfield & J. Roux, HPC 3501 (holotype CBS H-24800, culture ex-type CPC 40354 = CBS 148254).
Notes: Phaeosphaeria was treated by Marin-Felix et al. (2019). Phaeosphaeria hyphaenes is closely related (Fig. 2, 50) to P. phoenicicola (on Phoenix canariensis, New Zealand, conidia (8–) 12–14(–16) × (2–)2.5(–3) μm; Crous et al. 2016, Marin-Felix et al. 2019), but is distinct in that it has shorter conidia.
Fig. 50.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 of the Phaeosphaeria ITS nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Pleospora iqbalii (culture CBS 362.69; GenBank NR_160118.1) and the species treated here is highlighted with bold face. Accession numbers of sequence from material with a type status are also shown in bold face.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Phaeosphaeria sp. 1 NV-2015 [strain UTHSC DI16-336, GenBank LT796889.1; Identities = 507/511 (99 %), no gaps], Phaeosphaeria phoenicicola [strain CPC 28711, GenBank NR_156608.1; Identities = 502/512 (98 %), one gap (0 %)], and Hendersonia culmiseda [strain CBS 100.72, GenBank MH860403.1; Identities = 500/512 (98 %), one gap (0 %)]. Closest hits using the LSU sequence are Phaeosphaeria cycadis [strain KUMCC 18-0161, GenBank NG_070078.1; Identities = 808/808 (100 %), no gaps], Phaeosphaeria acaciae [voucher MFLU 17-0496, GenBank NG_069453.1; Identities = 808/808 (100 %), no gaps], and Phaeosphaeria lunariae [strain CPC 26679, GenBank KX306791.1; Identities = 808/808 (100 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Phaeosphaeria sp. 1 NV-2015 [strain UTHSC DI16-325, GenBank LT797042.1; Identities = 808/837 (97 %), no gaps], Phaeosphaeria caricis-sectae [strain CPC 38771, GenBank MZ078195.1; Identities = 763/837 (91 %), no gaps], and Phaeosphaeria oryzae [strain MFLUCC 11-0170, GenBank KM434306.1; Identities = 568/624 (91 %), no gaps]. No significant hits were obtained when the tef1 first part) sequence was used in blastn and megablast searches. Closest hits using the tub2 sequence had highest similarity to Phaeosphaeria oryzae [strain CBS 110110, GenBank KF252680.1; Identities = 220/234 (94 %), no gaps], Phaeosphaeria sp. 1 NV-2015 [strain UTHSC DI16-325, GenBank LT796962.1; Identities = 322/343 (94 %), four gaps (1 %)], and Phaeosphaeria podocarpi [strain CBS 138903, GenBank KP004508.1; Identities = 408/457 (89 %), 12 gaps (2 %)].
Authors: P.W. Crous, J.Z. Groenewald, J. Roux & M.J. Wingfield
Phlyctema phoenicis Crous & Thangavel, Persoonia 37: 349. 2016. Fig. 51.
Fig. 51.
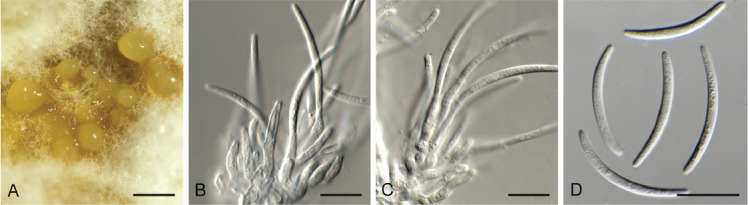
Phlyctema phoenicis (CPC 38747). A. Conidiomata on OA. B, C. Conidiogenous cells giving rise to conidia. D. Conidia. Scale bars: A = 300 μm, all others = 10 μm.
Taxonomic lineage: Leotiomycetes, Helotiales, Dermateaceae.
Conidiomata eustromatica, erumpent, acervuloid, separate, yellowish brown, pulvinate, round, unilocular, 200–300 μm diam; wall of brown textura angularis. Conidiophores hyaline, smooth, 1–6-septate, irregularly branched, cylindrical, filiform, 18–40 × 3–4 μm. Conidiogenous cells subcylindrical, hyaline, smooth, phialidic, terminal and intercalary, with minute collarette and periclinal thickening, 6–17 × 2.5–3 μm. Conidia hyaline, smooth, aseptate, fusoid, curved, ends subobtuse, guttulate, (20–)25–30(–43) × (2–)2.5 μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and lobate, even margin, reaching 50 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface dirty white to buff, reverse sienna.
38747
Material examined: New Zealand, Tauranga port, on Libertia ixioides (Iridaceae), 2019, C. Inglis, CBS H-24529, culture CPC 38747 = CBS 147066.
Notes: Phlyctema phoenicis was described from New Zealand, where it occurs on Phoenix canariensis, having conidia that are (17–)24–26(–27) × 2(–2.5) μm (Crous et al. 2016). Although the conidia of the holotype are smaller than those of the present collection on Libertia ixioides, the two collections are phylogenetically closely related (Fig 4).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Golovinomyces cynoglossi [strain 5_50, GenBank KY660948.1; Identities = 545/547 (99 %), no gaps], Rhabdospora lupini [strain CBS 475.69, GenBank MH859359.1; Identities = 544/546 (99 %), no gaps], and Phlyctema phoenicis [strain CPC 29372, GenBank NR_155690.1; Identities = 540/542 (99 %), no gaps]. Closest hits using the LSU sequence are Phlyctema coronillae [voucher MFLU 15-1243, GenBank MT449705.1; Identities = 857/858 (99 %), no gaps], Pseudofabraea citricarpa [strain WZ-12-C-Co, GenBank MK968360.1; Identities = 848/849 (99 %), no gaps], and Phlyctema vagabunda [strain CBS 261.32, GenBank MH866769.1; Identities = 876/878 (99 %), no gaps]. Distant hits obtained using the actA sequence had highest similarity to Coleophoma eucalyptigena [strain CBS 146018, GenBank MT223749.1; Identities = 474/529 (90 %), 13 gaps (2 %)], Satchmopsis brasiliensis [strain CPC 24855, GenBank MT432192.1; Identities = 389/420 (93 %), no gaps], and Satchmopsis pini [strain CBS 146687, GenBank MT375096.1; Identities = 398/435 (91 %), one gap (0 %)]. Closest hits using the tub2 sequence had highest similarity to Phlyctema phoenicis [strain CPC 29372, GenBank KY173611.1; Identities = 428/434 (99 %), no gaps], Neofabraea eucalyptorum [strain CBS 146634, GenBank MT375121.1; Identities = 374/454 (82 %), 14 gaps (3 %)], and Coleophoma xanthosiae [strain CPC 29214, GenBank KY173598.1; Identities = 234/269 (87 %), three gaps (1 %)].
Authors: P.W. Crous, J.Z. Groenewald & R. Thangavel
Pseudohormonema Crous, gen. nov. MycoBank MB 844306.
Taxonomic lineage: Dothideomycetes, Dothideales, incertae sedis.
Etymology: Resembling the genus Hormonema.
Mycelium consisting of golden brown, finely verruculose, thick-walled, mucoid, guttulate hyphae. Conidiophores reduced to conidiogenous loci on hyphae, intercalary, phialidic, with truncate locus, giving rise to solitary conidia that aggregate in mucoid mass. Conidia initially hyaline, smooth, aseptate, guttulate to granular, ellipsoid, with age becoming medianly septate, each cell dividing further, golden brown, finely verruculose, then elongating to form chains of such chlamydospore-like structures.
Type species: Pseudohormonema sordidus Crous
Pseudohormonema sordidus Crous, sp. nov. MycoBank MB 844307. Fig. 52.
Fig. 52.

Pseudohormonema sordidus (CBS 130468). A. Colony on SNA. B–D. Brown hyphae giving rise to hyaline, aseptate conidia. E. Hypha. F. Conidia becoming septate, brown, thick-walled, and anastomosing to form chains. G. Hyaline, aseptate conidia. Scale bars = 10 μm.
Etymology: Sordida (L.) dirty, impure, referring to its ecology.
Mycelium consisting of golden brown, finely verruculose, thick-walled, mucoid, guttulate, 5–7 μm diam hyphae. Conidiophores reduced to conidiogenous loci on hyphae, intercalary, phialidic, with truncate locus, 2 μm diam, giving rise to solitary conidia that aggregate in mucoid mass. Conidia initially hyaline, smooth, aseptate, guttulate to granular, ellipsoid, (6–)7–8(–10) × (3.5–) 4(–4.5) μm, apex obtuse, tapering in lower third to truncate hilum, 1–1.5 μm diam, with age becoming medianly septate, each cell dividing, conidium becoming 4–8-celled, golden brown, finely verruculose, then elongating to form chains of such microsclerotium-like structures, each 1–4 or more-septate, eventually giving rise to hyphae with intercalary conidiogenous cells that again form aseptate, hyaline conidia.
Culture characteristics: Colonies erumpent, spreading, slimy, smooth, even to lobate margins, aerial mycelium absent, reaching 5 mm diam after 7 d at 25 °C in the dark. On MEA, PDA and OA surface and reverse fuscous black.
Typus: USA, from human pacemaker, date unknown, D. Sutton (holotype CBS-H 25001, culture ex-type CBS 130468 = UTHSC 07-2004).
Additional materials examined: Saudi Arabia, from polluted soil, Aug. 2014, coll. S. de Hoog & T.A. Moussa, isol. M. Machado, cultures CBS 140365, CBS 140366.
Notes: Pseudohormonema represents a hormonema-like genus in Dothideales (see discussion under Dothiora above; Fig. 1 part 1), described as an opportunistic human pathogen, and from polluted soils. Although morphologically similar to Hormonema, it is different in that conidia become multiseptate, golden brown, finely verruculose, forming chains of microsclerotium-like structures in culture. Although the two strains from Saudi Arabia differ from the ex-type based on tub2 (see below), we for now refrain from introducing an additional species for those strains pending the collection of more isolates to determine the genetic diversity of the present species.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CBS 130468 had highest similarity to Sarcinomyces crustaceus [strain UAMH 4286, GenBank NR_121503.1; Identities = 535/615 (87 %), 27 gaps (4 %)], Zalaria alba [strain DAOM C250847, GenBank NR_153465.1; Identities = 531/613 (87 %), 22 gaps (3 %)], and Zalaria obscura [strain DAOM C250849, GenBank NR_153466.1; Identities = 529/611 (87 %), 22 gaps (3 %)]. The ITS sequence of CBS 130468 differs with one and two nucleotides respectively from CBS 140365 and 140366 (599/600 and 600/602 bp identical). Closest hits using the LSU sequence of CBS 130468 are Neodothiora populina [strain CPC 39399, GenBank MW175405.1; Identities = 549/570 (96 %), four gaps (0 %)], Neocylindroseptoria pistaciae [strain CBS 471.69, GenBank NG_057996.1; Identities = 549/572 (96 %), two gaps (0 %)], and Kabatina mahoniae [strain CBS 264.92, GenBank MH874022.1; Identities = 549/572 (96 %), four gaps (0 %)]. The LSU sequences of CBS 130468 and 140365 are identical (570/570 bp). No significant hits were obtained when the actA and tub2 sequences of CBS 130468 were used in a blast search. The tub2 sequence of CBS 130468 is 93 % similar to those of CBS 140365 and 140366 (496/532 bp and 494/529 bp, both including one gap)
Authors: P.W. Crous & J.Z. Groenewald
Pseudoplagiostoma eucalypti Cheew. et al., Fungal Diversity 44: 98. 2010. Fig. 53.
Fig. 53.
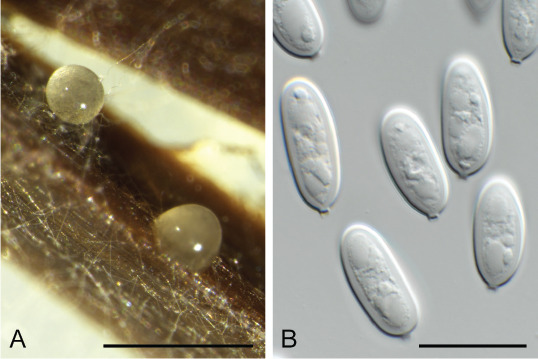
Pseudoplagiostoma eucalypti (CPC 39762). A. Conidiomata on PNA. B. Conidia. Scale bars: A = 300 μm, B = 10 μm.
Taxonomic lineage: Sordariomycetes, Diaporthales, Pseudoplagiostomataceae.
Description and illustration: Cheewangkoon et al. (2010).
Material examined: South Africa, KwaZulu-Natal Province, Kwambonambi, on leaves of Eucalyptus benthamii (Myrtaceae), 8 Jun. 2020, J. Roux, HPC 3444, culture CPC 39762 = CBS 149183.
Notes: Species of Pseudoplagiostoma (Pseudoplagiostomataceae, Diaporthales; Fig. 6) are common foliar pathogens of Eucalyptus (Cheewangkoon et al. 2010, Crous et al. 2019c). This is the first record of a Pseudoplagiostoma species occurring on Eucalyptus in South Africa.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Pseudoplagiostoma eucalypti [strain LTL560, GenBank MF663591.1; Identities = 603/603 (100 %), no gaps], Pseudoplagiostoma oldii [strain CBS 124808, GenBank GU973534.1; Identities = 565/568 (9 9%), two gaps (0 %)], and Pseudoplagiostoma corymbiicola [strain CBS 145052, GenBank NR_161119.1; Identities = 597/604 (99 %), two gaps (0 %)]. Closest hits using the LSU sequence are Pseudoplagiostoma eucalypti [strain CPC 12280, GenBank GU973601.1; Identities = 734/734 (100 %), no gaps], Pseudoplagiostoma oldii [strain CBS 124808, GenBank GU973609.1; Identities = 733/734 (99 %), no gaps], and Pseudoplagiostoma corymbiicola [strain CBS 145052, GenBank NG_066280.1; Identities = 813/816 (99 %), no gaps]. Closest hits using the tef1 (first part) sequence had highest similarity to Pseudoplagiostoma eucalypti [strain CPC 14161, GenBank GU973540.1; Identities = 321/325 (99 %), one gap (0 %)], Pseudoplagiostoma oldii [strain CBS 124808, GenBank GU973564.1; Identities = 319/325 (98 %), no gaps], and Pseudoplagiostoma corymbiicola [strain CBS 145052, GenBank MK047558.1; Identities = 485/523 (93 %), five gaps (0 %)]. Closest hits using the tub2 sequence had highest similarity to Pseudoplagiostoma eucalypti [strain PE1, GenBank KT831772.1; Identities = 731/731 (100 %), no gaps], Pseudoplagiostoma oldii [as Diaporthales sp. CR-2010a; strain CBS 124808, GenBank GU993862.1; Identities = 718/736 (98 %), one gap (0 %)], and Pseudoplagiostoma variabile [as Diaporthales sp. CR-2010b; strain CBS 113067, GenBank GU993863.1; Identities = 709/736 (96 %), one gap (0 %)].
Authors: P.W. Crous, J.Z. Groenewald & J. Roux
Pseudosoloacrosporiella cryptomeriae Crous, Persoonia 47: 195. 2021. Fig. 54.
Fig. 54.
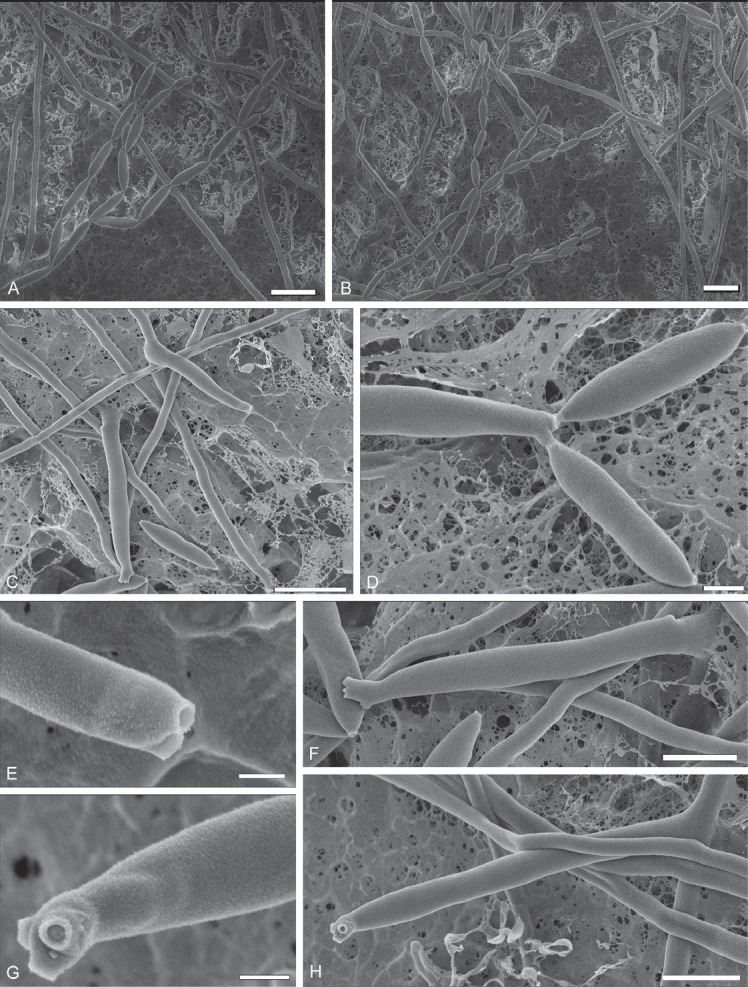
Pseudosoloacrosporiella cryptomeriae (CPC 39587). A, B. Scanning Electron Micrographs showing conidial chains. C–H. Conidiophores and conidiogenous cells showing rhexolytic conidiogenesis. Scale bars: A–C = 10 μm, F, H = 5 μm, D = 2 μm, E, G = 1 μm.
Taxonomic lineage: Dothideomycetes, Microthyriales, Microthyriaceae.
Description and illustration: Crous et al. (2021).
Material examined: Netherlands, Gelderland Province, Wageningen, Belmonte Botanical Garden, on leaves of Cryptomeria japonica (Cupressaceae), 28 July 2020, P.W. Crous, HPC 3301 (holotype CBS H-24883, culture ex-type CPC 39587 = CBS 148441).
Notes: Pseudoacrosporiella (Microthyriaceae, Microthyriales; Fig. 1 part 1) was recently introduced as a new genus of hyphomycetes resembling Soloacrosporiella, but being distinct in that it lacks setae, and its conidia do not have thickened and darkened hila, but rather have a characteristic marginal frill resulting from rhexolytic conidiogenesis (Crous et al. 2021d). The scanning electron micrographs clearly show this characteristic feature (Fig. 54).
Authors: P.W. Crous & J. Dijksterhuis
Pseudosydowia Thambug. & K.D. Hyde, Fungal Diversity 68: 140. 2014.
New synonym: Moringomyces Crous, Persoonia 45: 323. 2020.
Taxonomic lineage: Dothideomycetes, Dothideales, Saccotheciaceae.
Pseudosydowia phantasmae (Crous) Crous, comb. nov. MycoBank MB 845100. Fig. 55.
Fig. 55.
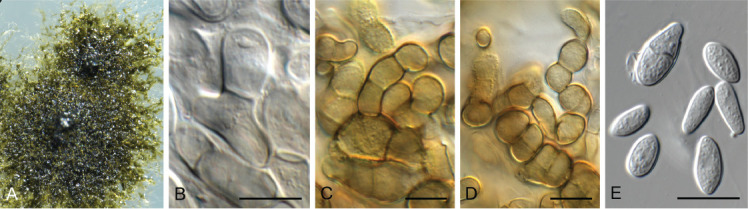
Pseudosydowia phantasmae (CPC 38950). A. Colony on SNA. B–D. Swollen hyphae that develop conidiogenous loci giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Basionym: Moringomyces phantasmae Crous, Persoonia 45: 323. 2020.
Conidiomata immersed on seed pods, pycnidial to acervular, 180–200 μm diam. In culture forming acervular conidiomata, arising from a brown stroma consisting of brown, smooth to verruculose, thin-walled cells, 5–8 μm diam. Conidiophores reduced to conidiogenous cells, ampulliform to doliiform, hyaline, smooth, mono- to polyphialidic, 7–10 × 10–14 μm. Conidia aggregating in mucoid mass, hyaline, smooth, 0(–1)-septate, ellipsoid to ovoid, frequently curved, becoming brown with age, apex subobtuse, base truncate, (9–)12–14(–15) 4–5 μm.
Material examined: Namibia, Namib Research Institute, on flower of Moringa ovalifolia (Moringaceae), 19 Nov. 2019, P.W. Crous, HPC 3130 = CBS H-24494, culture CPC 38950 = CBS 146982.
Culture characteristics: Colonies erumpent, spreading, surface folded, with sparse aerial mycelium and smooth, lobate margin, reaching 35 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse iron-grey.
Notes: Moringomyces phantasmae was recently described from Moringa ovalifolia in Namibia (Crous et al. 2020a), for which this isolate represents the second collection (Fig. 1 part 1). In previous analyses (Crous et al. 2020a, 2021a), Moringomyces phantasmae clustered distinct from Pseudosydowia. However, by including an additional isolate in the present analysis, Moringomyces is placed within Pseudosydowia (type species: P. eucalypti), albeit on a long branch with no support. Pseudosydowia is morphologically highly variable (Crous et al. 2019c), but includes the variation we see in Moringomyces, and hence the latter is reduced to synonymy.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Moringomyces phantasmae [strain CBS 146830, GenBank NR_171999.1; Identities = 603/604 (99 %), no gaps], Pseudosydowia eucalypti [strain CBS 131832, GenBank MH865934.1; Identities = 485/529 (92 %), 15 gaps (2 %)], and Cryptocline arctostaphyli [strain 19GCAS004, GenBank MW077685.1; Identities = 553/609 (91 %), 22 gaps (3 %)]. Closest hits using the LSU sequence are Moringomyces phantasmae [strain CPC 38883, GenBank MW175404.1; Identities = 834/834 (100 %), no gaps], Pseudosydowia eucalypti [strain CPC 14028, GenBank GQ303327.2; Identities = 837/855 (98 %), three gaps (0 %)], and Pseudosydowia eucalyptorum [strain CBS 145546, GenBank NG_067893.1; Identities = 830/848 (98 %), three gaps (0 %)].
Authors: P.W. Crous, J.Z. Groenewald, N. Yilmaz & M.J. Wingfield
Quasiphoma Crous, gen. nov. MycoBank MB 844308.
Taxonomic lineage: Dothideomycetes, Pleosporales, Leptosphaeriaceae.
Etymology: Name refers to the fact that it is phoma-like in morphology, but different, being allied to other phoma-like genera.
Conidiomata pycnidial, solitary, brown, globose, with central ostiole; wall of 3–6 layers of medium brown textura angularis; conidiomata exuding a creamy to pinkish conidial mass. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline smooth, ampulliform to doliiform, phialidic. Conidia solitary, or in short, false, disarticulating chains, hyaline, smooth- and thin-walled, guttulate, ellipsoid, with obtuse ends, aseptate.
Type species: Quasiphoma hyphaenes Crous
Quasiphoma hyphaenes Crous, sp. nov. MycoBank MB 844309. Fig. 56.
Fig. 56.
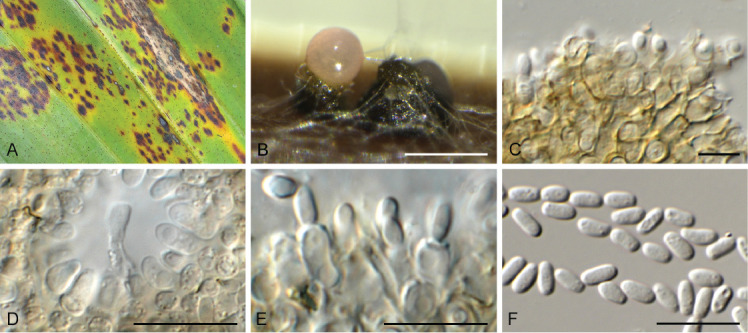
Quasiphoma hyphaenes (CPC 40045). A. Leaf spots on Hyphaene crenata. B. Conidiomata on PNA. C, E. Conidiogenous cells giving rise to conidia. D. Ostiole viewed from above. F. Conidia. Scale bars: B = 250 μm, all others = 10 μm.
Etymology: Name refers to the host genus Hyphaene from which it was isolated.
Conidiomata pycnidial, solitary, brown, globose, 200–250 μm diam, with central ostiole; wall of 3–6 layers of medium brown textura angularis; conidiomata exuding a creamy to pinkish conidial mass. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline smooth, ampulliform to doliiform, phialidic, 5–7 × 3–3.5 μm. Conidia solitary, or in short, false, disarticulating chains, hyaline, smooth- and thin-walled, guttulate, ellipsoid, with obtuse ends, aseptate, (4–)5(–6) × 2(–2.5) μm.
Culture characteristics: Colonies erumpent, spreading, with abundant aerial mycelium and smooth, lobate margin, covering dish within 2 wk at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: South Africa, KwaZulu-Natal Province, Manguzi, on leaves of Hyphaene sp. (Arecaceae), 19 Oct. 2017, M.J. Wingfield & J. Roux, HPC 3501 (holotype CBS H-24798, culture ex-type CPC 40045 = CBS 148253).
Notes: Based on preliminary analyses (data not shown) of ITS, LSU, rpb2 and tub2, Quasiphoma hyphaenes clustered between a reference strain of Coniothyrium telephii (characterised by setose pycnidia), and Querciphoma minuta (conidiomata are eustromatic, uni- to multi-locular, and conidia become brown and verruculose with age; Crous & Groenewald 2017). Quasiphoma hyphaenes is a nondescript phoma-like taxon, except that at times conidia appear to remain attached in short chains, which is unusual. It is associated with Querciphoma and Subplenodomus in the LSU phylogeny (Fig. 2).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Phoma schachtii [strain CBS 502.84, GenBank MH861770.1; Identities = 430/469 (92 %), 11 gaps (2 %)], Longiseptatispora curvata [strain CPC 36457, GenBank NR_169725.1; Identities = 427/467 (91 %), nine gaps (1 %)], and Coniothyrium wernsdorffiae [strain CBS 150.34, GenBank MH855474.1; Identities = 428/469 (91 %), 12 gaps (2 %)]. Closest hits using the LSU sequence are Subplenodomus drobnjacensis [strain PRG11-03, GenBank AB819763.1; Identities = 795/814 (98 %), no gaps], Alternariaster helianthi [strain CBS 119672, GenBank KC584368.1; Identities = 795/815 (98 %), four gaps (0 %)], and Querciphoma minuta [as Coniothyrium carteri; strain LG1401_MS6E, GenBank KX359604.1; Identities = 794/814 (98 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Coniothyrium telephii [strain CBS 188.71, GenBank KT389593.1; Identities = 512/598 (86 %), no gaps], Exserohilum minor [as Setosphaeria minor; strain BRIP 14615, GenBank LT852501.1; Identities = 662/812 (82 %), two gaps (0 %)], and Exserohilum rostratum [as Setosphaeria rostrata; strain BRIP 13592, GenBank LT882528.1; Identities = 661/813 (81 %), four gaps (0 %)]. No significant hits were obtained when the actA and tub2 sequences were used in blastn and megablast searches.
Authors: P.W. Crous, J.Z. Groenewald, J. Roux & M.J. Wingfield
Racheliella wingfieldiana Crous & U. Braun, Fungal Syst. Evol. 1: 71. 2018. Fig. 57.
Fig. 57.
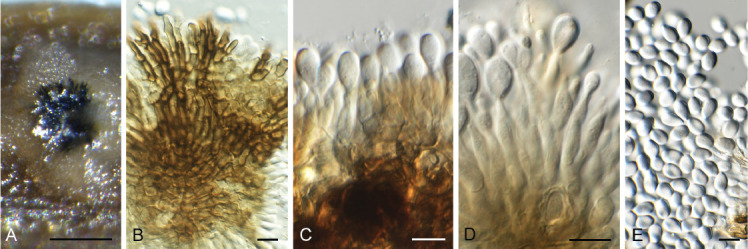
Racheliella wingfieldiana (CPC 40039). A. Pycnothyrium on PNA. B. Radiating wall of pycnothyrium. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Sordariomycetes, Diaporthales, Tubakiaceae.
Description and illustration: Crous et al. (2018b).
Material examined: South Africa, near Mozambique, on Syzygium sp. (Myrtaceae), 19 Oct. 2015, M.J. Wingfield, HPC 3498 = CBS H-24796, culture CPC 40039 = CBS 148251.
Notes: Racheliella wingfieldiana (Tubakiaceae, Diaporthales; Fig. 6) was described from leaves of Syzygium guineense in the Eastern Cape Province of South Africa (Crous et al. 2018b). The present collection appears to represent a closely related cryptic lineage, but more isolates are required to determine the genetic variation within R. wingfieldiana.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Racheliella wingfieldiana [strain CBS 143669, GenBank MG591911.1; Identities = 575/583 (99 %), two gaps (0 %)], Paratubakia subglobosa [strain ATCC 22474, GenBank NR_161043.1; Identities = 375/420 (89 %), 17 gaps (4 %)], and Oblongisporothyrium castanopsidis [strain CBS 124732, GenBank MG591849.1; Identities = 373/419 (89 %), 16 gaps (3 %)]. Closest hits using the LSU sequence are Racheliella wingfieldiana [strain CBS 143669, GenBank MG592006.1; Identities = 805/805 (100 %), no gaps], Greeneria saprophytica [strain NTCL052-1, GenBank KJ021935.1; Identities = 810/815 (99 %), no gaps], and Apiognomonioides supraseptata [strain CBS 632.92, GenBank NG_066205.1; Identities = 808/815 (99 %), no gaps]. The best hit using the tef1 (first part) sequence had highest similarity to Racheliella wingfieldiana [strain CBS 143669, GenBank MG592100.1; Identities = 579/588 (98 %), three gaps (0 %)]; no other significant hits were obtained. The best hit using the tub2 sequence had highest similarity to Racheliella wingfieldiana [strain CBS 143669, GenBank MG592192.1; Identities = 418/432 (97 %), one gap (0 %)]; no other significant hits were obtained.
Authors: P.W. Crous, J.Z. Groenewald & M.J. Wingfield
Ramularia arvensis Sacc., Michelia 2(no. 8): 548. 1882. Fig. 58.
Fig. 58.
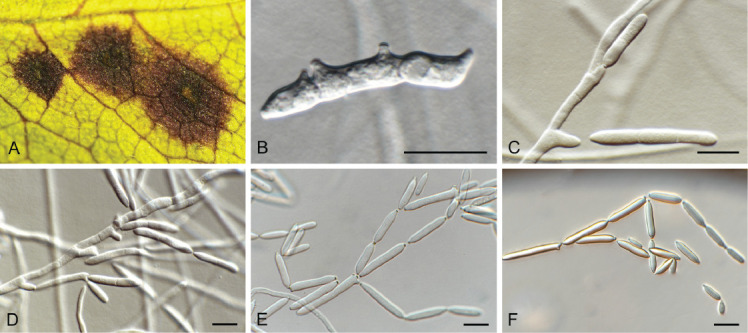
Ramularia arvensis (CPC 39985). A. Leaf spots. B. Ramoconidium. C, D. Conidiophores giving rise to conidia. E, F. Catenulate conidia. Scale bars = 10 μm.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Mycosphaerellaceae.
Leaf spots amphigenous, circular, brown to dark brown, 2–4 mm diam. Conidiophores fasciculate, amphigenous. On SNA. Mycelium consisting of hyaline, smooth, branched, septate, 2.5–3.5 μm diam hyphae. Conidiophores reduced to conidiogenous cells on hyphae, solitary, erect, subcylindrical, 5–25 × 3–4 μm; loci terminal, thickened, darkened, refractive, 1–1.5 μm diam. Ramoconidia subcylindrical, hyaline, smooth-walled, 1–3-septate, 20–55 × 3.5–4.5 μm; conidia in branched chains, subcylindrical, hyaline, smooth-walled, (0–)1-septate, (10–)15–20(–30) × 3(–4) μm; hila thickened, darkened, refractive, 1 μm diam.
Culture characteristics: Colonies erumpent, surface folded, with moderate aerial mycelium and smooth, lobate margin, reaching 10 mm diam after 7 d at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: Italy, Selva, Treviso, on Potentilla reptans (Rosaceae) (holotype, herb. Saccardo PAD). Netherlands, Gelderland Province, Beuningen, river Waal, on leaves of Potentilla reptans, 17 Oct. 2020, A.L. van Iperen, HPC 3475 (epitype designated here CBS H-24897, MBT 10007416, culture ex-epitype CPC 39985 = CBS 148455).
Notes: The present collection was initially thought to be R. grevilleana, which has a broad host range and distribution. The latter species was treated in detail by Braun (1998) who also listed several synonyms described from Europe on Potentilla (e.g. R. anserina, R. arvensis and R. punctiformis). Phylogenetically (Fig. 1 part 2, 13 part 2) and morphologically however, our collection is distinct from R. grevilleana (on Fragaria spp.), having branched conidial chains, ramoconidia, and smaller subcylindrical terminal conidia. The oldest name which can be applied to this specimen is Ramularia arvensis, described from Italy on Potentilla reptans. One yet older name exists (Ramularia martianoffiana), described from Asia, Siberia on another Asian Potentilla species, but the type has not been preserved, and its morphology cannot be confirmed.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Ramularia collo-cygni [strain CBS 101180, GenBank NR_154944.1; Identities = 516/522 (99 %), three gaps (0 %)], Ramularia glennii [strain SFI-D21, GenBank MT535837.1; Identities = 517/524 (99 %), three gaps (0 %)], and Ramularia eucalypti [strain SFI-D21, GenBank MT535837.1; Identities = 517/524 (99 %), three gaps (0 %)]. Closest hits using the LSU sequence are Ramularia uredinicola [strain CPC 12491, GenBank KX287226.1; Identities = 809/812 (99 %), no gaps], Ramularia bellunensis [strain CBS 118417, GenBank KX287035.1; Identities = 824/828 (99 %), one gap (0 %)], and Ramularia lamii var. lamii [strain CBS 108970, GenBank NG_069361.1; Identities = 817/822 (99 %), one gap (0 %)]. Closest hits using the actA sequence had highest similarity to Ramularia grevilleana [strain CBS 719.84, GenBank KP894331.1; Identities = 587/617 (95 %), 10 gaps (1 %)], Ramularia eucalypti [strain CPC 13044, GenBank KJ504458.1; Identities = 560/610 (92 %), 20 gaps (3 %)], and Ramularia glennii [strain CBS 129441, GenBank KJ504433.1; Identities = 557/609 (91 %), 19 gaps (3 %)]. Closest hits using the gapdh sequence had highest similarity to Ramularia grevilleana [strain CBS 259.36, GenBank KP894549.1; Identities = 505/524 (96 %), no gaps], Ramularia pratensis var. pratensis [strain CPC 19448, GenBank KX288330.1; Identities = 423/464 (91 %), six gaps (1 %)], and Ramularia hydrangeae-macrophyllae [strain CPC 25903, GenBank KX288295.1; Identities = 421/463 (91 %), seven gaps (1 %)]. Closest hits using the his3 sequence had highest similarity to Ramularia grevilleana [strain CBS 259.36, GenBank KP894771.1; Identities = 339/356 (95 %), eight gaps (2 %)], Ramularia vallisumbrosae [strain CBS 271.38, GenBank KX288988.1; Identities = 332/357 (93 %), four gaps (1 %)], and Ramularia pratensis [strain CBS 136.23, GenBank KJ504633.1; Identities = 328/356 (92 %), 14 gaps (3 %)]. Closest hits using the tef1 (first part) sequence had highest similarity to Ramularia grevilleana [strain CBS 114732, GenBank KP894438.1; Identities = 304/320 (95 %), no gaps], Ramularia rumicicola [strain CPC 11294, GenBank KX288063.1; Identities = 172/186 (92 %), four gaps (2 %)], and Ramularia haroldporteri [strain CPC 16296, GenBank KJ504681.1; Identities = 150/153 (98 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald, U. Braun, M. Starink-Willemse & A.L. van Iperen
Rapidomyces Crous & Boers, gen. nov. MycoBank MB 844310.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Teratosphaeriaceae.
Etymology: Rapid-, relating to the rate of ascomatal development in damp chambers.
Ascomata substomatal, immersed to superficial, solitary, brown, linked by brown hyphal network, globose to subglobose, with central ostiole; wall of 2–3 layers of brown textura angularis. Asci 8-spored, broadly ellipsoid to subglobose, bitunicate. Ascospores multiseriate, hyaline, smooth-walled, guttulate, fusoid-ellipsoid, medianly 1-septate, constricted at septum. Ascospores becoming brown and verruculose upon discharge, with prominent mucoid sheath.
Type species: Rapidomyces narthecii Crous & Boers
Rapidomyces narthecii Crous & Boers, sp. nov. MycoBank MB 844311. Fig. 59.
Fig. 59.
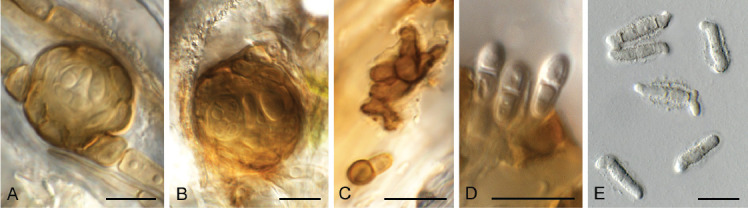
Rapidomyces narthecii (CPC 41974). A. B. Superficial ascomata. C. Superficial ascospores on host tissue. D. Ascospores. E. Germinating ascospores. Scale bars = 10 μm.
Etymology: Name refers to the genus Narthecium from which it was isolated.
Ascomata substomatal, immersed to superficial, solitary, brown, linked by brown hyphal network, 5–6 μm diam, globose to subglobose, 15–40 μm diam, with central ostiole; wall of 2–3 layers of brown textura angularis (developing in damp chambers from superficial hyphae on host tissue within 3–5 d of incubation). Asci 8-spored, broadly ellipsoid to subglobose, bitunicate, 10–20 × 8–12 μm. Ascospores multiseriate, hyaline, smooth-walled, guttulate, fusoid-ellipsoid, medianly 1-septate, constricted at septum, widest just above septum, 8–9 × 3–3.5 μm. Ascospores becoming brown and verruculose upon discharge, with prominent mucoid sheath; ascospores 4 μm diam, germinating from polar ends, with germ tubes at various angles to the long axis of the spore.
Culture characteristics: Colonies spreading, with moderate aerial mycelium and even, lobate margins, reaching 15 mm diam after 2 wk. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: Netherlands, Drenthe Province, Dwingelderveld National Park, 52.829188, 6.432495, on dead leaves of Narthecium ossifragum (Nartheciaceae), 4 Jul. 2021, J. Boers, HPC 2353 (holotype CBS H-24997, cultures ex-type CPC 41974, 41975 = CBS 149174).
Notes: Rapidomyces represents a new, phylogenetically distinct sexual genus in Teratosphaeriaceae (Fig. 1 part 2, 36). It is related to several extremophilic genera, namely Lapidomyces (rock inhabiting hyphomycetous fungus; Crous et al. 2019a), Xenopenidiella formica (ant inhabiting hyphomycetous fungus; Duarte et al. 2017), Acrodontium crateriforme (hyphomycete occurring on various substrates; Videira et al. 2016), Pseudotaeniolina (rock inhabiting hyphomycete; De Leo et al. 2003), and Neocatenulostroma (saprobic and plant pathogenic; Crous et al. 2007a). Morphologically, it lacks an asexual morph, and resembles other sexual genera in Teratosphaeriaceae.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Pseudotaeniolina globosa [strain CBS 109889, GenBank MH862844.1; Identities = 382/420 (91 %), four gaps (0 %)], Teratosphaeria jonkershoekensis [strain CBS 122897, GenBank EU707864.1; Identities = 378/415 (91 %), seven gaps (1 %)], and Neocatenulostroma microsporum [strain HFJN1, GenBank MH349092.1; Identities = 374/413 (91 %), four gaps (0 %)]. Closest hits using the LSU sequence are Neocatenulostroma germanicum [strain CBS 539.88, GenBank MH873835.1; Identities = 797/835 (95 %), 10 gaps (1 %)], Aulographina pinorum [strain CBS 302.71, GenBank GU214393.1; Identities = 797/835 (95 %), 10 gaps (1 %)], and Neocatenulostroma microsporum [strain CBS 110890, GenBank EU019255.2; Identities = 797/835 (95 %), 10 gaps (1 %)]. Closest hits using the rpb2 sequence had distant similarity to Teratosphaeria fibrillosa [strain CPC 13960, GenBank LT799765.1; Identities = 558/711 (78 %), 24 gaps (3 %)], Ramularia chamaedryos [strain CBS 116577, GenBank KX288512.1; Identities = 360/456 (79 %), four gaps (0 %)], and Collarispora valgourgensis [strain CBS 129531, GenBank MF951479.1; Identities = 359/460 (78 %), seven gaps (1 %)].
Authors: P.W. Crous, J.Z. Groenewald & J. Boers
Reticulascus parahennebertii Crous & Osieck, sp. nov. MycoBank MB 844312. Fig. 60.
Fig. 60.
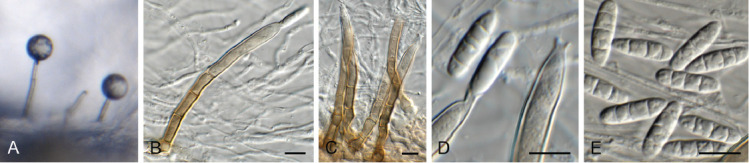
Reticulascus parahennebertii (CPC 41226). A. Conidiophores on SNA. B–D. Conidiophores and conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Taxonomic lineage: Sordariomycetes, Glomerellales, Reticulascaceae.
Etymology: Refers to its morphological similarity to Reticulascus hennebertii.
Mycelium consisting of hyaline, smooth-walled, branched, septate, 2.5–3 μm diam hyphae, frequently forming hyphal coils. Conidiophores solitary or in clusters, subcylindrical, unbranched, medium brown, thick-walled, straight to flexuous, base bulbous, 6–8 μm diam, 3–4-septate, intermingled among sterile setae, similar in morphology but with obtuse apical cells, 50–100 × 4–5 μm. Conidiogenous cells terminal, intercalary, subcylindrical, pale brown, smooth, with apical monophialides, 35–45 μm long, with flared collarettes, 3–4 μm diam. Conidia solitary, aggregating in mucoid mass, subcylindrical, hyaline, smooth, guttulate, apex obtuse, tapering to truncate hilum, 1.5–2 μm diam, 3-septate, (12–)13–14(–15) × (3.5–)4 μm.
Culture characteristics: Colonies flat, spreading, surface folded, with moderate aerial mycelium and smooth, lobate margin, reaching 25 mm diam after 2 wk at 25 °C. On MEA surface and reverse smoke grey; on PDA surface pale smoke grey, reverse pale smoke grey with dirty white margin; on OA surface smoke grey.
Material examined: Netherlands, Utrecht Province, Nieuw Wulven, near Houten, 52°02’44’’N, 5°09’40’’E, 1.5 m a.s.l., on dead culm of Juncus inflexus (Juncaceae), 4 Feb. 2021, E.R. Osieck, HPC 3590 = WI-21/#4205 (holotype CBS H-24828, culture ex-type CPC 41226 = CBS 148282).
Notes: Réblová et al. (2011) reduced Cylindrotrichum hennebertii (CBS 570.76) to synonymy under Reticulascus tulasneorum (asexual morph C. oligospermum) (ex-type strain CBS 101319). Cylindrotrichum hennebertii was described as having conidia that are variable in size, 0–3-septate, 7–13 × 2–3 μm, and lacking sterile setae in vivo (Gams & Holubová-Jechová 1976). Reticulascus parahennebertii is distinct in having slightly wider conidia and sterile setae, which also resolves it as a distinct species of Reticulascus (Fig. 7).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to “Cylindrotrichum sp.” [strain 1552, GenBank AM262411.1; Identities = 451/452 (99 %), no gaps], Blastophorum aquaticum [strain MFLUCC 15-0264, GenBank NR_153629.1; Identities = 487/548 (89 %), 14 gaps (2 %)], and Cylindrotrichum submersum [voucher MFLU 18-2320, GenBank NR_168818.1; Identities = 481/541 (89 %), 10 gaps (1 %)]. Closest hits using the LSU sequence are Reticulascus tulasneorum [strain CBS 101319, GenBank AF178547.2; Identities = 790/805 (98 %), two gaps (0 %)], Cylindrotrichum submersum [voucher MFLU 18-2320, GenBank NG_068641.1; Identities = 758/776 (98 %), two gaps (0 %)], and Chaetopsis grisea [strain CBS 100.57, GenBank MH869199.1; Identities = 790/810 (98 %), seven gaps (0 %)]. Closest hits using the tef1 (second part) sequence had highest similarity to Leptosillia pistaciae [strain ISPaVe 1958, GenBank MK523320.1; Identities = 801/888 (90 %), two gaps (0 %)], Cylindrotrichum submersum [voucher MFLU 18-2320, GenBank MN200280.1; Identities = 741/823 (90 %), two gaps (0 %)], and Leptosillia macrospora [strain CRM2, GenBank MK523314.1; Identities = 794/888 (89 %), two gaps (0 %)].
Authors: P.W. Crous, J.Z. Groenewald & E.R. Osieck
Rhexocercosporidium bellocense (C. Massal. & Sacc.) Crous, J. Kruse & U. Braun, comb. nov. MycoBank MB 844313. Fig. 61.
Fig. 61.
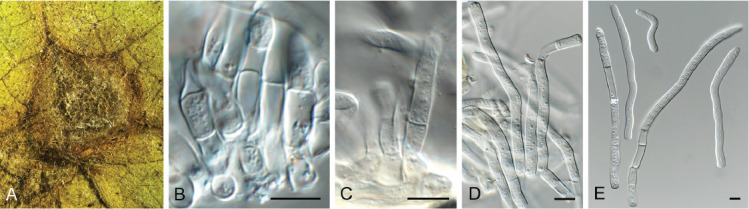
Rhexocercosporidium bellocense (CPC 39764). A. Leaf spot on Verbascum cf. densiflorum. B–D. Conidiophores and conidiogenous cells giving rise to conidia. E. Conidia in disarticulating chains. Scale bars = 10 μm.
Basionym: Septocylindrium bellocense C. Massal. & Sacc., Annls mycol. 6: 558. 1908.
Synonym: Thedgonia bellocensis (C. Massal. & Sacc.) U. Braun, Nova Hedwigia 54(3–4): 471. 1992.
Taxonomic lineage: Leotiomycetes, Helotiales, Ploettnerulaceae.
Leaf spots variable, subcircular, amphigenous, pale to medium brown, 2–10 mm diam, margin dark brown, diffuse. Conidiomata fasciculate, predominantly epiphyllous, whitish on leaf surface. Conidiophores arising from poorly developed brownish stroma, in dense fascicles, 20–60 × 3–6 μm, straight to geniculate-sinuous, subcylindrical, 1–2-septate, subhyaline. Conidiogenous cells terminal, integrated, holothallic, rarely sympodial, scars unthickened, 7–20 × 3–4 μm; conidiogenous cells difficult to discern from conidia. Conidia in disarticulating chains, cylindrical, apex obtuse, base truncate, (20–)50–100(–160) × 3.5–6 μm, hyaline, smooth, 3–6(–10)-septate; hila not thickened nor darkened.
Culture characteristics: Cultures sterile. Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 20 mm diam after 7 d at 25 °C. On MEA surface and reverse olivaceous grey; on PDA surface olivaceous grey, reverse iron grey; on OA surface iron grey.
Typus: Italy, Verona, M. Belloca, on Verbascum nigrum (Scrophulariaceae), 1908, Massalongo (holotype VER). Germany, Rhineland-Palatinate, Bad Kreuznach, 49°48’13”N, 7°44’8”E, on leaves of Verbascum cf. densiflorum , 14 Jun. 2020, J. Kruse, HPC 3451 (epitype designated here, CBS H-24836, MBT 10007419; isoepitype POLL 9787; culture ex-epitype CPC 39764 = CBS 148297).
Notes: Septocylindrium bellocense was described from leaves of Verbascum nigrum collected in Italy, for which the present collection from Germany is an appropriate specimen to serve for epitypification. Braun (1995) noted conidia to be catenate, (20–) 30–130(–200) × 3–7 μm, 3–20-septate, sometimes even more, and occurring on different Verbascum spp. in Europe. Because S. bellocense has hyaline conidia occurring in chains, Braun (1992) placed it in Thedgonia. However, it is not congeneric with the type of Thedgonia (T. ligustrina; Leotiomycetes), but clusters with Rhexocercosporidium carotae, the type of Rhexocercosporidium (Fig. 4). The latter genus was introduced to accommodate Arothecium carotae, a pathogen of carrots, characterised by hyaline to subhyaline, solitary conidia. As shown here, however, species of Rhexocercosporidium can also have conidia arranged in chains.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Rhexocercosporidium carotae [strain Rc1-18Dc, GenBank MN594496.1; Identities = 467/486 (96 %), no gaps], Phialophora cinerescens [strain CBS 280.35, GenBank MH855676.1; Identities = 475/497 (96 %), no gaps], and Polyscytalum pustulans [strain PP3, GenBank EU196535.1; Identities = 474/498 (95 %), no gaps]. Closest hits using the LSU sequence are Rhexocercosporidium panacis [strain C22-K9, GenBank MN385763.1; Identities = 758/764 (99 %), no gaps], Cadophora constrictospora [strain P2414, GenBank MN339394.1; Identities = 757/764 (99 %), no gaps], and Cadophora luteo-olivacea [strain P2092, GenBank MN339390.1; Identities = 757/764 (99 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Cadophora cf. interclivum [strain P6081, GenBank MN367269.1; Identities = 531/583(91%), no gaps], Rhexocercosporidium panacis [strain C22-K9, GenBank MN787159.1; Identities = 770/852 (90 %), no gaps], and Rhexocercosporidium carotae [strain C22-K8, GenBank MN787158.1; Identities = 770/852 (90 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald, J. Kruse & U. Braun
Rhexocercosporidium aff. bellocense. Fig. 62.
Fig. 62.
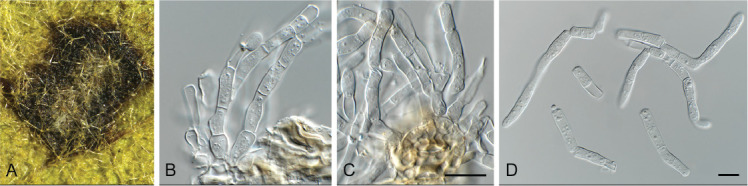
Rhexocercosporidium aff. bellocensis (CPC 39690). A. Leaf spot on Verbascum sp. B, C. Conidiophores and conidiogenous cells giving rise to conidia. D. Conidia in disarticulating chains. Scale bars = 10 μm.
Taxonomic lineage: Leotiomycetes, Helotiales, Ploettnerulaceae.
Leaf spots amphigenous, subcircular, 2–5 mm diam, dark purple, with indistinct border. Conidiomata fasciculate, amphigenous, predominantly whitish on leaf surface. Conidiophores arising from stromata that are erumpent, pale brown, up to 60 μm diam; conidiophores subcylindrical, straight to geniculate-sinuous, 1–4-septate, hyaline, 12–50 × 6–7 μm. Conidiogenous cells terminal, integrated, with flat, unthickened scars, 12–25 × 5–6 μm. Conidia solitary in disarticulating chains, cylindrical, straight to curved, apex obtuse, base truncate, (20–)70–100(–160) × 5(–6) μm, hyaline, smooth, 1–11-septate; hilum unthickened, nor darkened.
Culture characteristics: Cultures sterile. Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 17 mm diam after 7 d at 25 °C. On MEA, PDA and OA surface dirty white and reverse olivaceous grey.
Material examined: Russia, Rostov region, Krasnosulinsky district, state natural wildlife area “Gornensky”, disturbed steppe near road, on leaves of Verbascum sp. (Scrophulariaceae), 13 Jun. 2020, T.S. Bulgakov, HPC 3414 = PC 032 = CBS H-24791 = LE F-332411, culture CPC 39690 = CBS 148246.
Notes: The Russian collection of Rhexocercosporidium aff. bellocense is morphologically similar to R. bellocense, but differs in having somewhat wider conidiophores, and shorter and wider conidia compared to the description of R. bellocense in Braun (1995), which was based on measurements of a number of collections on Verbascum spp. in Europe [conidiophores 20–60 × 3–6 μm, conidia (20–)30–130(–200) × 3–7 μm, 3–20-septate]. The Russian collection might well fall into the variability of R. bellocense, above all, since the two species are genetically highly similar (based on a comparison with CPC 39764; ITS: 831/844 bp, LSU: 762/764 bp, rpb2: 873/874 bp, respectively similar; Fig. 4), but cryptic speciation can also not be excluded with certainty. However, a final conclusion is not yet possible, based on a single specimen and culture.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Rhexocercosporidium carotae [strain Rc2-18Dc, GenBank MN594497.1; Identities = 546/574 (95 %), two gaps (0 %)], Phialophora cinerescens [strain CBS 280.35, GenBank MH855676.1; Identities = 557/586 (95 %), two gaps (0 %)], and Cadophora luteo-olivacea [strain STAF242, GenBank KU214556.1; Identities = 554/584 (95 %), one gap (0 %)]. Closest hits using the LSU sequence are Rhexocercosporidium panacis [strain C22-K9, GenBank MN385763.1; Identities = 814/818 (99 %), no gaps], Cadophora luteo-olivacea [strain CBS 128572, GenBank MH878015.1; Identities = 813/818 (99 %), no gaps], and Cadophora malorum [strain CBS 117865, GenBank MH874580.1; Identities = 813/818 (99 %), no gaps]. Closest hits using the rpb2 sequence had highest similarity to Cadophora cf. interclivum [strain P6081, GenBank MN367269.1; Identities = 531/583 (91 %), no gaps], Cadophora cf. meredithiae [strain P1313, GenBank MN367229.1; Identities = 552/612 (90 %), no gaps], and Rhexocercosporidium panacis [strain C22-K9, GenBank MN787159.1; Identities = 772/856 (90 %), no gaps]. Closest hits using the tub2 sequence had highest similarity to Pyrenopeziza brassicae [strain PbFr002, GenBank KC342227.1; Identities = 432/505 (86 %), 14 gaps (2 %)], Cadophora meredithiae [strain BAG2, GenBank MF677914.1; Identities = 421/493 (85 %), 18 gaps (3 %)], and Rhexocercosporidium panacis [strain RP17, GenBank MT822282.1; Identities = 383/450 (85 %), seven gaps (1 %)].
Authors: P.W. Crous, J.Z. Groenewald, J. Kruse & U. Braun
Rhopographus filicinus (Fr.) Nitschke ex Fuckel, Jb. nassau. Ver. Naturk. 23–24: 219. 1870 (1869–1870). Fig. 63.
Fig. 63.
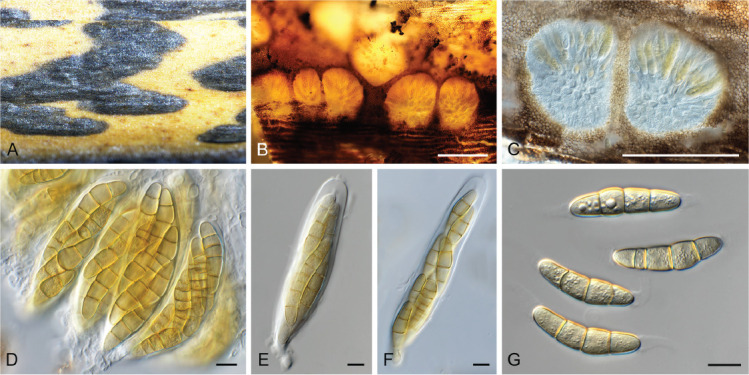
Rhopographus filicinus (CPC 41597). A. Erumpent stroma with ascomata. B, C. Section though stroma, showing ascomatal cavities. D–F. Asci. G. Ascospores. Scale bars: B, C = 200 μm, all others = 10 μm.
Basionym: Sphaeria filicina Fr., Syst. Mycol. (Lundae) 2: 427. 1823 [replaced synonym Sphaeria pteridis Sowerby, Col. Fig. Engl. Fung. Mushr. (London) 3(no. 27): tab. 394, fig. 10. 1803, designated as lectotype here, MBT 10007421].
Taxonomic lineage: Dothideomycetes, Pleosporales, Didymellaceae.
Stromata linear, subepidermal, black, glossy, 0.5–3 mm diam wide, 1 mm to several cm long, becoming confluent, forming conspicuous crusts on dead petioles. Ascomata immersed in linear rows in stroma, opening via linear split with periphyses at apex, globose, 120–220 μm diam, wall of 8–15 layers of medium brown textura angularis to textura globulosa. Pseudoparaphyses intermingled among asci, hyaline, branched, septate, anastomosing, 2–3 μm diam, constricted at septa, hyphae-like. Asci bitunicate, 8-spored straight to slightly curved, subcylindrical to narrowly cymbiform, apex obtuse, apical chamber 1.5–2 μm diam, pedicellate, 70–95 × 15–18 μm. Ascospores tri- to multiseriate, golden brown, smooth, granular, fusoid-ellipsoid with obtuse ends, straight to slightly curved, initially 3-septate, constricted at medium septum, swollen in cell above medium septum, 3(–7)-septate, septa darker brown, with mucoid appendages at both ends, up to 15 μm long, (26–)28–33(–36) × (6–)7–8(–9) μm.
Typus: Netherlands, Utrecht Province, Bilthoven, on dead leaf fronds of Pteridium aquilinum (Hypolepidaceae), Jun. 2021, P.W. Crous, HPC 3632 (epitype designated here CBS H-24998, MBT 10007422, culture ex-epitype CPC 41596, 41597 = CBS 149224).
Additional material examined: Netherlands, Utrecht Province, Lage Vuursche, on stem of Pteridium aquilinum, 13 Jun. 2021, P.W. Crous, HPC 3645, cultures CPC 41925, 41926.
Notes: Rhopographus filicinus is the type species of the genus Rhopographus, which has to date had an unresolved higher phylogeny, being a member of Dothideomycetes (Didymellaceae, Pleosporales; Fig. 2). Rhopographus filicinus causes black, raised mosaic lesions on stems of Pteridium aquilinum, and is common in the Netherlands, and probably also elsewhere in Europe.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 41596 had highest similarity to Rhopographus filicinus [strain CBS 384.59, GenBank MH857898.1; Identities = 514/514 (100 %), no gaps], Phoma herbarum [strain CBS 368.61, GenBank MH858087.1; Identities = 483/518 (93 %), eight gaps (1 %)], and Ascochyta orobi [strain CBS 105.30, GenBank MH855088.1; Identities = 483/518 (93 %), eight gaps (1 %)]. The ITS sequences of CPC 41596 and 41925 are identical. Closest hits using the LSU sequence of CPC 41596 are Didymella rumicicola [strain CBS 683.79, GenBank NG_069775.1; Identities = 794/805 (99 %), no gaps], Didymella macrophylla [strain CGMCC 3.18357, GenBank NG_069441.1; Identities = 793/805 (99 %), no gaps], and Didymella aquatica [strain CGMCC 3.18349, GenBank NG_069439.1; Identities = 793/805 (99 %), no gaps]. The LSU sequences of CPC 41596 and 41925 are identical to each other and also to the LSU sequence of CBS 384.59 generated in this study. Closest hits using the actA sequence of CPC 41596 had distant similarity to species in Didymellaceae, e.g. Boeremia exigua var. pseudolilacis [strain CPC 25089, GenBank KT193804.1; Identities = 552/630 (88 %), 16 gaps (2 %)], Ascochyta rabiei [as Didymella rabiei; strain AR628, GenBank KM244530.1; Identities = 551/629 (88 %), 16 gaps (2 %)], and Calophoma sandfjordenica [strain CBS 145571, GenBank MK876453.1; Identities = 553/630 (88 %), 16 gaps (2 %)]. The actA sequences of CPC 41596 and 41925 are identical. No significant hits were obtained when the rpb2 sequence of CPC 41596 was used in blastn and megablast searches.
Authors: P.W. Crous, J.Z. Groenewald, M. Starink-Willemse & A.L. van Iperen
Russula lilacina (A.H. Sm.) Trappe & T.F. Elliott, comb. nov. MycoBank MB 821382.
Basionym: Macowanites lilacinus A.H. Sm., Mycologia 55: 426. 1963.
Taxonomic lineage: Agaricomycetes, Agaricales, Russulaceae.
Description and illustration: Smith (1963).
Notes: In light of efforts to resolve the nomenclatural issues surrounding sequestrate generic names in the Russulaceae, Elliott & Trappe (2018a, b) combined all members of Bucholtzia, Cystangium, Elasmomyces, Gymnomyces, Macowanites, and Martellia with the traditionally agaricoid genus Russula. However, Macowanites lilacinus was overlooked. We here combine the last remaining member of the genus Macowanites with Russula and name it: Russula lilacina (A.H. Smith) Trappe & T.F. Elliott. To see the justification for this combination, please review Elliott & Trappe (2018a).
Authors: T.F. Elliott & J.M. Trappe
Scytalidium philadelphianum Crous & Jurjević, sp. nov. MycoBank MB 844315. Fig. 64.
Fig. 64.
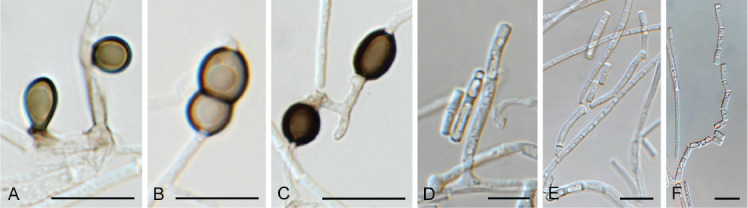
Scytalidium philadelphianum (CPC 40793). A–C. Chlamydospores. D–F. Conidial chains. Scale bars = 10 μm.
Taxonomic lineage: Leotiomycetes, Helotiales, Helotiaceae.
Etymology: Name refers to the location where it was isolated, Philadelphia, Pennsylvania.
Mycelium consisting of hyaline, smooth-walled, branched, septate, 2–2.5 μm diam hyphae. Conidiophores erect, solitary, subcylindrical, straight to flexuous, unbranched, 1–2-septate, 10–30 × 2.5–4 μm. Conidiogenous cells integrated, terminal, subcylindrical, hyaline, smooth, 10–15 × 3–4 μm, holoblastic, proliferating sympodially at apex, which is slightly swollen; scars inconspicuous, truncate, 2–3 μm diam. Conidia occurring in long disarticulating conidial chains, hyaline, smooth-walled, guttulate, subcylindrical, not constricted at septa, segments 3–6 × (2–)2.5–3 μm. Chlamydospores dark brown, globose to ellipsoid, 0–1-septate, 4–6 μm diam, thick-walled, smooth to roughened, intercalary to terminal, in terminal, frequently adjacent to erect conidiophores.
Culture characteristics: Colonies flat, spreading, with abundant aerial mycelium, covering dish after 7 d at 25 °C. On MEA, CYA (Czapek yeast agar), PDA and OA surface and reverse iron grey. On CYA covering dish after 7 d at 37 °C.
Typus: USA, Pennsylvania, Philadelphia, from compressed air in a factory, Oct. 2020, Ž. Jurjević 5560 (holotype CBS H-24808, culture ex-type CPC 40793 = CBS 148262).
Notes: Scytalidium philadelphianum is congeneric to S. lignicola, but clusters distinct from the ex-type isolate CBS 233.57 = UAMH 1502 (Fig. 65). Morphologically it is similar, but distinct in that it has wider conidia and 0–1-septate, narrower chlamydospores (arthroconidia 5–8 × 2 μm, chlamydospores up to 7 μm wide; Pesante 1957).
Fig. 65.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 (Minh et al. 2020) of the Scytalidium ITS nucleotide alignment. Bootstrap support values (> 69 % are shown; only values > 94 % are significant) from 5 000 ultrafast bootstrap replicates are shown at the nodes. Culture collection numbers and GenBank accession numbers (superscript) are indicated for all species. The tree was rooted to Rhizodermea veluwensis (culture CBS 110605; GenBank NR_165956.1) and the species treated here is highlighted with bold face. Accession numbers of sequence from material with a type status are also shown in bold face.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Scytalidium lignicola [strain UAMH 1502, GenBank NR_121314.1; Identities = 548/581 (94 %), 16 gaps (2 %)], Scytalidium cuboideum [as Arthrographis cuboidea; GenBank AY557369.1; Identities = 483/544 (89 %), 25 gaps (4 %)], and Trichoderma afroharzianum [voucher research collection Farrer lab 122, GenBank MN644645.1; Identities = 526/596 (88 %), 39 gaps (6 %)].
Authors: P.W. Crous, J.Z. Groenewald & Ž. Jurjević
Septoria chelidonii (Lib.) Desm., Annls Sci. Nat., Bot., sér. 2 17: 110. 1842. Fig. 66.
Fig. 66.
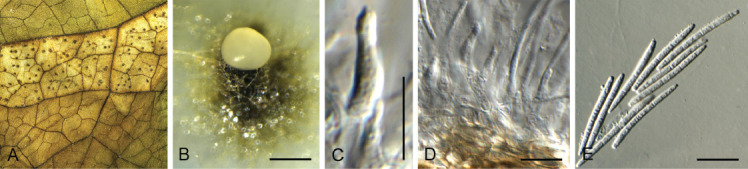
Septoria chelidonii (CPC 39746). A. Leaf spot with conidiomata. B. Conidioma on OA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars: B = 250 μm, all others = 10 μm.
Basionym: Ascochyta chelidonii Lib., Pl. crypt. Arduenna, fasc. (Liège) 3(nos 201–300): no. 204. 1834.
Synonym: Spilosphaeria chelidonii (Lib.) Rabenh., Klotzschii Herb. Viv. Mycol., Edn Nov, Ser. Sec., Cent. 6: no. 552. 1857.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Mycosphaerellaceae.
Leaf spots amphigenous, angular, elongated, 2–4 mm diam, medium brown with raised, dark brown border. Conidiomata pycnidial, brown, erumpent, 200–250 μm diam, opening by central ostiole; wall of 2–3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells or with a supporting cell, branched or not, 7–15 × 3–4 μm, hyaline, smooth. Conidiogenous cells terminal or lateral, ampulliform, hyaline, smooth, proliferating percurrently near apex, 7–10 × 3–4 μm. Conidia solitary, hyaline, smooth, guttulate, subcylindrical, straight to slightly curved, apex subobtuse, base truncate, (1–)3-septate, (21–)26–29(–35) × 2(–2.5) μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and feathery, lobate margin, reaching 15 mm diam after 7 d at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Material examined: Russia, Rostov region, Krasnosulinsky district, state natural wildlife area “Gornensky”, roadside of artificial forest road, on Chelidonium majus (Papaveraceae), 10 Jul. 2020, T.S. Bulgakov, HPC 3326 = CBS H-24898 = PC-010 = LE F-332406, culture CPC 39746 = CBS 148456.
Notes: Septoria chelidonii (Mycosphaerellaceae, Myco-sphaerellales; Figs 1 part 2, 13 part 1) is a well-known pathogen of Chelidonium spp. (Verkley et al. 2013), and has previously been reported from Russia (Mel’nik et al. 2008).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Septoria chelidonii [strain CBS 132027, GenBank GU269860.1; Identities = 493/493 (100 %), no gaps], Septoria cerastii [strain CBS 128626, GenBank MH865054.1; Identities = 506/510 (99 %), no gaps], Septoria sigesbeckiae [strain CBS 128661, GenBank MH865108.1; Identities = 529/534 (99 %), no gaps], and Septoria perillae [strain CBS 128655, GenBank MH865103.1; Identities = 529/534 (99 %), no gaps]. Closest hits using the LSU sequence are Septoria senecionis [strain CBS 102381, GenBank NG_069162.1; Identities = 802/803 (99 %), no gaps], Septoria galeopsidis [strain CBS 102411, GenBank NG_069160.1; Identities = 802/803 (99 %), no gaps], and Septoria epilobii [strain CBS 109085, GenBank NG_069159.1; Identities = 802/803 (99 %), no gaps]. Closest hits using the actA sequence had highest similarity to Septoria chelidonii [strain CBS 128607, GenBank KF253676.1; Identities = 214/214 (100 %), no gaps], Septoria gaurina [strain QD-3, GenBank MW187076.1; Identities = 558/566 (99 %), no gaps], Septoria cerastii [strain CBS 132028, GenBank JQ325030.1; Identities = 546/556 (98 %), four gaps (0 %)], and Septoria melissae [voucher KACC47728, GenBank MH359403.1; Identities = 573/586 (98 %), one gap (0 %)]. Closest hits using the tef1 (first part) sequence had highest similarity to Septoria chelidonii [strain CBS 128607, GenBank KF253319.1; Identities = 330/331 (99 %), no gaps], Septoria gaurina [strain QD-3, GenBank MW187078.1; Identities = 326/333 (98 %), two gaps (0 %)], and Septoria agrimoniicola [strain CBS 128585, GenBank KF253283.1; Identities = 325/333 (98 %), two gaps (0 %)].
Authors: P.W. Crous & J.Z. Groenewald
Septoria robiniae (Lib.) Desm., Annls Sci. Nat., Bot., sér. 3 11: 349. 1849. Fig. 67.
Fig. 67.
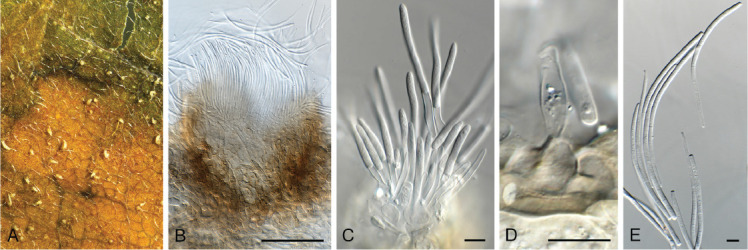
Septoria robiniae (CPC 39783). A. Leaf spot with conidiomata. B. Conidioma with exuding conidial mass. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars: B = 60 μm, all others = 10 μm.
Basionym. Ascochyta robiniae Lib., Pl. crypt. Arduenna, fasc. (Liège) 4(nos 301–400): no. 357. 1837.
Synonyms. Phloeospora robiniae (Lib.) Höhn., Annls mycol. 3: 333. 1905.
Cylindrosporium robiniae (Lib.) Died., Krypt.-Fl. Brandenburg (Leipzig) 9: 846. 1915.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Mycosphaerellaceae.
Leaf spots amphigenous, medium brown, with diffuse chlorotic borders, 5–10 mm diam. Conidiomata amphigenous, acervular, 50–120 μm diam, immersed in epidermis, giving rise to a pale creamy conidial cirrhus; wall of 3–4 layers of pale brown textura angularis. Conidiophores reduced to conidiogenous cells, hyaline to pale brown, smooth-walled, ampulliform to subcylindrical, 6–12 × 5–7 μm, proliferating inconspicuously percurrently at apex. Conidia solitary, hyaline, smooth-walled, granular to guttulate, straight to curved, subcylindrical to acicular, apex subobtuse, base truncate to long obconically truncate, hilum with minute marginal frill, 1–2(–3)-septate, in vivo (36–)45–55(–66) × (3–)4(–4.5) μm, in vitro 65–90 × 3(–3.5) μm. The isotype (BR-MYCO 147039-84) had leaf spots that were amphigenous, subcircular to irregular, medium brown with diffuse margins, 4–8 mm diam. Conidiomata were similar to that of the epitype. Conidiogenous cells 7–10 × 3.5–5 μm, and conidia subcylindrical to acicular, 1–2(–3)-septate, (28–)38–55(–60) × (3–)3.5–4 μm.
Culture characteristics: Colonies erumpent, with sparse aerial mycelium and feathery, lobate margin, reaching 5 mm diam after 7 d at 25 °C. On MEA surface and reverse umber; on PDA surface and reverse olivaceous grey; on OA surface olivaceous grey.
Typus: Belgium, on leaves of Robinia pseudoacacia (Fabaceae), exsicata M.A. Libert – Pl. Crypt. Arduenna fasc. (Liège) 4: 357 (1837), designated here as lectotype, BR-MYCO 147039-84, MBT 10007424; Limburg, Heusden-Zolder, on leaves of R. pseudoacacia, 22 Jul. 2020, C. Van Steenwinkel, HPC 3454 (epitype designated here CBS H-24839, MBT 10007425; culture ex-epitype CPC 39783 = CBS 148300).
Notes: Septoria robiniae (as Phloeospora robiniae) is an important foliar pathogen of Robinia pseudoacacia in Europe, causing leaf necrosis and deformation from early spring onwards (Kehr & Butin 1996). Because this pathogen produces prominent acervuli with erect, creamy conidial cirrhi, it has for long been assumed to be a member of Phloeospora. However, Verkley et al. (2013) demonstrated that species of Septoria could have conidiomatal pycnidia and/or acervuli, thus this feature alone is insufficient to separate members of this complex. The present collection closely matches the morphology of the holotype, and thus it is a good choice for typification to fix the genetic application of the name (Mycosphaerellaceae, Mycosphaerellales; Figs 1 part 2, 13 part 1).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Septoria apocyni [strain LB-02, GenBank KR611856.1; Identities = 503/505 (99 %), no gaps], Septoria pistaciarum [strain SE, GenBank MZ268218.1; Identities = 513/516 (99 %), no gaps], and Septoria posoniensis [strain CBS 128645, GenBank MH865067.1; Identities = 512/516 (99 %), one gap (0 %)]. Closest hits using the LSU sequence are Apseudocercosporella trigonotidis [strain CBS 131890, GenBank NG_069100.1; Identities = 788/789 (99 %), no gaps], Septoria scabiosicola [strain CBS 356.58, GenBank MH869343.1; Identities = 788/789 (99 %), no gaps], and Septoria stellariae [strain CBS 102378, GenBank KF252080.1; Identities = 788/789 (99 %), no gaps]. Closest hits using the actA sequence had highest similarity to Septoria protearum [strain T19_05709B, GenBank MW890033.1; Identities = 536/580 (92 %), 11 gaps (1 %)], Septoria citri [strain CBS 315.37, GenBank JX902161.1; Identities = 498/541 (92 %), 11 gaps (2 %)], and Septoria carvi [strain KML1860, GenBank KX822110.1; Identities = 485/527 (92 %), four gaps (0 %)]. Closest hits using the tef1 (first part) sequence had highest similarity to Septoria rumicum [strain CBS 503.76, GenBank KF253478.1; Identities = 368/418 (88 %), 18 gaps (4 %)], Septoria pistaciarum [strain CPC 23116, GenBank KF442635.1; Identities = 433/514 (84 %), 24 gaps (4 %)], and Septoria hippocastani [strain CBS 411.61, GenBank KF253383.1; Identities = 363/417 (87 %), nine gaps (2 %)].
Authors: P.W. Crous, J.Z. Groenewald & C. Van Steenwinkel
Teratosphaeria alcornii Crous, Persoonia 23: 114. 2009. Fig. 68.
Fig. 68.
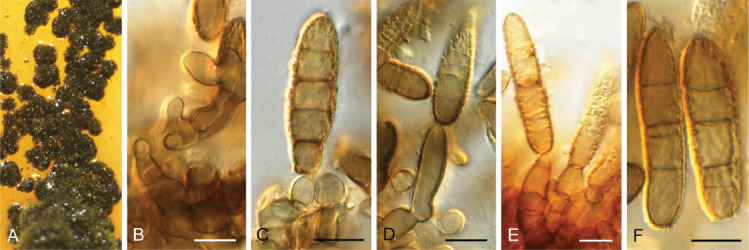
Teratosphaeria alcornii (CPC 39789). A. Colonies on MEA. B–E. Conidiogenous cells giving rise to conidia. F. Conidia. Scale bars = 10 μm.
Basionym: Stigmina eucalypti Alcorn, Trans. Br. mycol. Soc. 60: 151. 1973.
Taxonomic lineage: Dothideomycetes, Mycosphaerellales, Teratosphaeriaceae.
Descriptions and illustrations: Crous et al. (2009, 2019).
Material examined: South Africa, KwaZulu-Natal Province, Kwambonambi, on leaves of Corymbia citriodora (Myrtaceae), 9 Jun. 2020, J. Roux, HPC 3448, culture CPC 39789 = CBS 149184.
Notes: Teratosphaeria alcornii (Teratosphaeriaceae, Myco-sphaerellales; Fig. 1 part 2) is generally considered to be a minor pathogen on commercially propagated eucalypt hosts, although it can cause serious leaf spots on some species in Australia (Crous et al. 2009, 2019). This is the first record of T. alcornii occurring in South Africa.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Teratosphaeria alcornii [strain CPC 13384, GenBank EF394866.1; Identities = 477/478 (99 %), no gaps], Teratosphaeria pseudocryptica [strain CPC 29430, GenBank MN162082.1; Identities = 477/508 (94 %), 11 gaps (2 %)], and Teratosphaeria rubida [strain CBS 124579, GenBank MH863388.1; Identities = 476/507 (94 %), 11 gaps (2 %)]. Closest hits using the LSU sequence are Teratosphaeria alcornii [strain CBS 313.76, GenBank NG_066153.1; Identities = 803/803 (100 %), no gaps], Teratosphaeria complicata [strain CPC 14535, GenBank GQ852714.1; Identities = 793/801 (99 %), one gap (0 %)], and Teratosphaeria ovata [strain CPC 14632, GenBank FJ493218.1; Identities = 793/801 (99 %), one gap (0 %)]. Closest hits using the actA sequence had highest similarity to Teratosphaeria alcornii [strain CBS 121100, GenBank KF903646.1; Identities = 530/533 (99 %), no gaps], Teratosphaeria corymbiicola [strain CBS 146047, GenBank MN556788.1; Identities = 517/578 (89 %), 15 gaps (2 %)], and Teratosphaeria dunnii [strain CBS 145548, GenBank MK876463.1; Identities = 513/852 (88 %), 15 gaps (2 %)]. Closest hits using the cmdA sequence had highest similarity to Teratosphaeria alcornii [strain CBS 121100, GenBank KF902698.1; Identities = 384/388 (99 %), no gaps], Teratosphaeria angophorae [strain CBS 120493, GenBank KF902699.1; Identities = 346/388 (89 %), one gap (0 %)], and Teratosphaeria fimbriata [strain CBS 120736, GenBank KF902720.1; Identities = 344/388 (89 %), one gap (0 %)]. Closest hits using the rpb2 sequence had highest similarity to Teratosphaeria fimbriata [strain CPC 13324, GenBank LT799766.1; Identities = 612/671 (91 %), no gaps], Teratosphaeria cryptica [strain CBS 111663, GenBank KX348101.1; Identities = 728/845 (86 %), no gaps], and Teratosphaeria corymbiicola [strain CBS 146047, GenBank MN556802.1; Identities = 727/845 (86 %), no gaps. Closest hits using the tub2 sequence had highest similarity to Teratosphaeria alcornii [strain CBS 121100, GenBank KF902982.1; Identities = 246/251 (98 %), no gaps], Teratosphaeria molleriana [strain CBS 111164, GenBank KF252755.1; Identities = 239/278 (86 %), 15 gaps (5 %)], and Teratosphaeria considenianae [strain CBS 120087, GenBank FJ952510.1; Identities = 285/333 (86 %), 13 gaps (3 %)].
Authors: P.W. Crous, J.Z. Groenewald & J. Roux
Acknowledgments
We thank T.S. Bulgakov (Department of Plant Protection, Russian Academy of Sciences, Russia), for making several collections available for study.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Supplementary Material: http://fuse-journal.org/
Statistics for the different phylogenetic analyses performed in this study.
REFERENCES
- Alvarez LV, Groenewald JZ, Crous PW. (2016). Revising the Schizoparmaceae: Coniella and its synonyms Pilidiella and Schizoparme. Studies in Mycology 85: 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral HO, Weber E, Marson G. (2020). Monograph of Orbiliomycetes (Ascomycota) based on vital taxonomy. Part I + II. National Museum of Natural History; Luxembourg. [Google Scholar]
- Begoude BAD, Slippers B, Wingfield MJ. et al. (2010). Botryosphaeriaceae associated with Terminalia catappa in Cameroon, South Africa and Madagascar. Mycological Progress 9: 101–123. [Google Scholar]
- Bensch K, Braun U, Groenewald JZ, et al. (2012). The genus Cladosporium. Studies in Mycology 72: 1–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K, Groenewald JZ, Meijer M, et al. (2018). Cladosporium species in indoor environments. Studies in Mycology 89: 177–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerema GH, de Gruyter J, Noordeloos ME. (1997). Contributions towards a monograph of Phoma (Coelomycetes) - IV. Section Heterospora: Taxa with large sized conidial dimorphs, in vivo sometimes as Stagonosporopsis synanamorphs. Persoonia 16: 335–371 [Google Scholar]
- Braun U. (1992). Studies on Ramularia and allied genera (V). Nova Hedwigia 54: 459–478. [Google Scholar]
- Braun U. (1993). Taxonomic notes on some species of the Cercospora complex (II). Cryptogamic Botany 3: 235–244. [Google Scholar]
- Braun U. (1995). A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes): Vol. 1. IHW-Verlag, Eching, Munich, Germany. [Google Scholar]
- Braun U. (1998). A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes): Vol. 2. IHW-Verlag, Eching, Munich, Germany. [Google Scholar]
- Braun U, Hill CF. (2002). Some new micromycetes from New Zealand. Mycological Progress 1: 19–30. [Google Scholar]
- Braun U, Nakashima C, Crous PW, et al. (2018). Phylogeny and taxonomy of the genus Tubakia s. lat. Fungal Systematics and Evolution 1: 41–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubák F. (1916). Systematische Untersuchungen einiger, Farne bewohnenden Pilze. Berichte der Deutschen Botanischen Gesellschaft 34: 295–332. [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Verkley GJM, et al. (2010). Re-evaluation of Cryptosporiopsis eucalypti and Cryptosporiopsis-like species occurring on Eucalyptus. Fungal Diversity 44: 89–105. [Google Scholar]
- Chen C, Verkley GJM, Sun G, et al. (2016). Redefining common endophytes and plant pathogens in Neofabraea, Pezicula, and related genera. Fungal Biology 120: 1291–1322. [DOI] [PubMed] [Google Scholar]
- Chen Q, Jiang JR, Zhang GZ, et al. (2015). Resolving the Phoma enigma. Studies in Mycology 82: 137–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW. (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1–170. APS Press, MN, USA. [Google Scholar]
- Crous PW, Barreto RW, Alfenas AC, et al. (2010). What is Johansonia? IMA Fungus 1: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. (2007a). Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Cowan DA, Maggs-Kölling G, et al. (2020a). Fungal Planet description sheets: 1112–1181. Persoonia 45: 251–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Cowan DA, Maggs-Kölling G, et al. (2021a). Fungal Planet description sheets: 1182–1283. Persoonia 46: 313–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ. (2017). The Genera of Fungi – G4: Camarosporium and Dothiora. IMA Fungus 8: 131–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Wingfield MJ, et al. (2007b). Neofusicoccum mediterraneum Crous, M.J. Wingf. & A.J.L. Phillips, sp. nov. Fungal Planet 19: 1–2. [Google Scholar]
- Crous PW, Hernández-Restrepo M, Schumacher RK, et al. (2021b). New and Interesting Fungi. 4. Fungal Systematics and Evolution 7: 255–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Lombard L, Sandoval-Denis M, et al. (2021c). Fusarium: more than a node or a foot-shaped basal cell. Studies in Mycology 98: 100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Luangsa-ard JJ, Wingfield MJ, et al. (2018a). Fungal Planet description sheets: 785–867. Persoonia 41: 238–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Osieck ER, Jurjević Ž, et al. (2021d). Fungal Planet description sheets: 1284–1382. Persoonia 47: 178–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Akulov A, et al. (2019a). New and Interesting Fungi. 2. Fungal Systematics and Evolution 3: 57–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. (2018b). New and interesting fungi. 1. Fungal Systematics and Evolution 1: 169–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, et al. (2009). Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. (2011). Fungal Planet description sheets: 92–106. Persoonia 27: 130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, et al. (eds) (2019b). Fungal Biodiversity. [Westerdijk Laboratory Manual Series No. 1.]. Utrecht: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands. [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. (2016). Fungal Planet description sheets: 469–557. Persoonia 37: 218–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. (2017). Fungal Planet description sheets: 558–624. Persoonia 38: 240–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. (2018c). Fungal Planet description sheets: 716–784. Persoonia 40: 240–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Cheewangkoon R, et al. (2019c). Foliar pathogens of eucalypts. Studies in Mycology 94: 125–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. (2015). Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. (1991). Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628–632. [Google Scholar]
- Crous PW, Wingfield MJ, Schumacher RK, et al. (2020b). New and Interesting Fungi 3. Fungal Systematics and Evolution 6: 157–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Hernández-Restrepo M, Schumacher RK, et al. (2021e). New and Interesting Fungi. 4. Fungal Systematics and Evolution 7: 255–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog GS. (1985). Taxonomy of the Dactylaria complex, IV. Dactylaria, Neta, Subulispora and Scolecobasidium. Studies in Mycology 26: 1–60. [Google Scholar]
- De Leo F, Urzì C, de Hoog GS. (2003). A new meristematic fungus, Pseudotaeniolina globosa. Antonie Van Leeuwenhoek 83: 351–360. [DOI] [PubMed] [Google Scholar]
- Dingley JM. (1965). New records of fungous diseases on plants in New Zealand, 1962–64. New Zealand Journal of Agricultural Research 8: 905–920. [Google Scholar]
- Döbbeler P. (1978). Moosbewohnende Ascomyceten I. Die pyrenocarpen, den gametophyten besiedelnden arten. Mitteilungen der Botanischen Staatssammlung München 14: 1–360. [Google Scholar]
- Döbbeler P. (2007). Ascomycetes on Polytrichadelphus aristatus (Musci). Mycological Research 111: 1406–1421. [DOI] [PubMed] [Google Scholar]
- Duarte APM, Attili-Angelis D, Baron NC, et al. (2017). Riding with the ants. Persoonia 38: 81–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott TF, Trappe JM. (2018a). A worldwide nomenclature revision of sequestrate Russula species. Fungal Systematics and Evolution 1: 229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott TF, Trappe JM. (2018b). Erratum to: A worldwide nomenclature revision of sequestrate Russula species. Fungal Systematics and Evolution 2: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson OE. (1981). The families of bitunicate ascomycetes. Opera Botanica 60: 1–220. [Google Scholar]
- Fan XL, Barreto RW, Groenewald JZ, et al. (2017). Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes). Studies in Mycology 87: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XL, Bezerra JDP, Tian CM, et al. (2018). Families and genera of diaporthalean fungi associated with canker and dieback of tree hosts. Persoonia 40: 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gams W, Holubová-Jechová V. (1976). Chloridium and some other dematiaceous hyphomycetes growing on decaying wood. Studies in Mycology 13: 1–99. [Google Scholar]
- Gams W, Stielow B, Gräfenhan T, et al. (2019). The ascomycete genus Niesslia and associated monocillium-like anamorphs. Mycological Progress 18: 5–76. [Google Scholar]
- Goh TK, Hyde KD. (1997). A revision of Dactylaria, with description of D. tunicata sp. nov. from submerged wood in Australia. Mycological Research 101: 1265–1272. [Google Scholar]
- Gräfenhan T, Schroers H-J, Nirenberg HI, et al. (2011). An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Studies in Mycology 68: 79–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang DT, Chernomor O, von Haeseler A, et al. (2018). UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Holm K. (1977). A study of the Leptopeltidaceae. Botaniska Notiser 130: 215–229. [Google Scholar]
- Hongsanan S, Hyde KD, Phookamsak R, et al. (2020a). Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 11: 1553–2107. [Google Scholar]
- Hongsanan S, Hyde KD, Phookamsak R, et al. (2020b). Refined families of Dothideomycetes: orders and families incertae sedis in Dothideomycetes. Fungal Diversity 105: 17–318. [Google Scholar]
- Hyde KD, Norphanphoun C, Abreu VP, et al. (2017). Fungal diversity notes 603–708: taxonomic and phylogenetic notes on genera and species. Fungal Diversity 87: 1–235. [Google Scholar]
- Jenkins AE, Bitancourt AA. (1942). An Elsinoe causing an anthracnose of Virginia Creeper. Phytopathology 32: 424–427. [Google Scholar]
- Jenkins AE, Bitancourt AA. (1948). A spot anthracnose of flowering dogwood. Plant Disease Reporter 32: 253–255. [Google Scholar]
- Jülich W, de Vries B. (1982). On the genera Ascocorticium and Ascosorus (Ascocorticiaceae). Persoonia 11: 407–420. [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, et al. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods 14: 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr R, Butin H. (1996). Blattkrankheiten der Robinie. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes 48: 197–200. [Google Scholar]
- Klaubauf S, Tharreau D, Fournier E, et al. (2014). Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Studies in Mycology 79: 85–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisai-Greilhuber I, Chen Y, Jabeen S, et al. (2017). Fungal Systematics and Evolution: FUSE 3. Sydowia 69: 229–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Felix Y, Hernández-Restrepo M, Iturrieta-González I, et al. (2019). Genera of phytopathogenic fungi: GOPHY 3. Studies in Mycology 94: 1–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovskaja S, Kačergius A. (2014). Morphological and molecular characterisation of Periconia pseudobyssoides sp. nov. and closely related P. byssoides. Mycological Progress 13: 291–302. [Google Scholar]
- Mel’nik VA, Shabunin DA, Popov ES. (2008). Contributions to the studies of mycobiota in Novgorod and Pskov regions. II. Coelomycetes. Mikologiya I Fitopatologiya 42: 43–52. [Google Scholar]
- Minh BQ, Schmidt HA, Chernomor O, et al. (2020). IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37: 1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L-T, Schmidt HA, von Haeseler A, et al. (2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesante A. (1957). Osservazioni su una carie del platana. Annali della Sperimentazione Agaria 11 (suppl.): 249–266. [Google Scholar]
- Phukhamsakda C, McKenzie EHC, Phillips AJL, et al. (2020). Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Diversity 102: 1–203. [Google Scholar]
- Réblová M, Gams W, Seifert KA. (2011). Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Studies in Mycology 68: 163–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M, Hernández-Restrepo M, Fournier J, et al. (2020). New insights into the systematics of Bactrodesmium and its allies and introducing new genera, species and morphological patterns in the Pleurotheciales and Savoryellales (Sordariomycetes). Studies in Mycology 95: 415–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH. (1963). New astrogastraceous fungi from the Pacific Northwest. Mycologia 55: 421–441. [Google Scholar]
- Smith H, Wingfield MJ, Crous PW, et al. (1996). Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. South African Journal of Botany 62: 86–88. [Google Scholar]
- Stielow JB, Lévesque CA, Seifert KA, et al. (2015). One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 35: 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YP, Crous PW, Shivas RG. (2018). Cryptic species of Curvularia in the culture collection of the Queensland Plant Pathology Herbarium. MycoKeys 35: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkley GJM, Mel’nik VA, Shin H-D, et al. (2005). Camarographium koreanum sp. nov., a new coelomycete from Korea. Sydowia 57: 259–266. [Google Scholar]
- Verkley GJM, Quaedvlieg W, Shin HD, et al. (2013). A new approach to species delimitation in Septoria. Studies in Mycology 75: 213–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Braun U, et al. (2016). All that glitters is not Ramularia. Studies in Mycology 83: 49–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, et al. (2017). Mycosphaerellaceae – chaos or clarity? Studies in Mycology 87: 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Friebes G, Gardiennet A, et al. (2018). Barrmaelia and Entosordaria in Barrmaeliaceae (fam. nov., Xylariales) and critical notes on anthostomella-like genera based on multigene phylogenies. Mycological Progress 17: 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D, Groenewald M, de Vries M, et al. (2019). Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wäli PP, Huhtinen S, Pino-Bodas R, et al. (2014). Three common bryophilous fungi with meristematic anamorphs and phylogenetic alliance to Teratosphaeriaceae, Capnodiales. Fungal Biology 118: 956–969. [DOI] [PubMed] [Google Scholar]
- Wehmeyer LE. (1946). Studies on some fungi from northwest Wyoming. II. Fungi Imperfecti. Mycologia 38: 306–330. [PubMed] [Google Scholar]
- Zhang W, Groenewald JZ, Lombard L, et al. (2021). Evaluating species in Botryosphaeriales. Persoonia 46: 63–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Schwartz S, Wagner L, et al. (2000). A greedy algorithm for aligning DNA sequences. Journal of Computational Biology 7: 203–214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistics for the different phylogenetic analyses performed in this study.