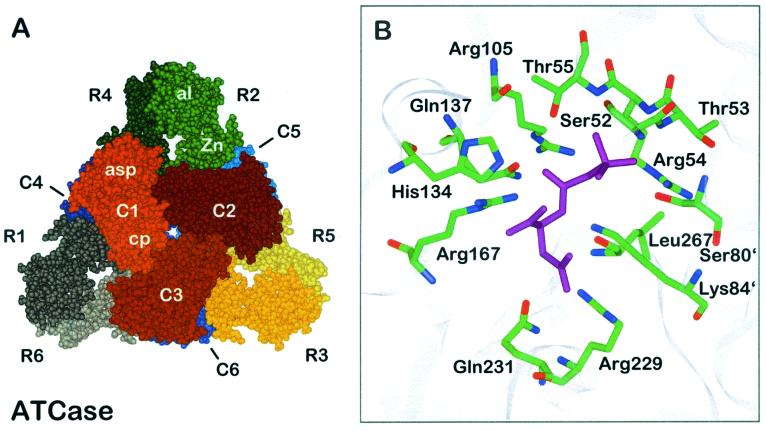
FIG. 1.
Quaternary structure of E. coli ATCase. (A) Holoenzyme viewed along the threefold axis. Catalytic chains are numbered C1 to C6, and regulatory chains are numbered R1 to R6. The different catalytic and regulatory subunits are indicated by different colors. The aspartate domain of the catalytic chain is designated asp, and the carbamoylphosphate domain is designated cp. The domains of the regulatory chain are named Zn for zinc domain and al for allosteric domain. (B) Binding mode of the bisubstrate analogue PALA (purple) to the active site of ATCase. Side chains are shown as sticks with atoms labeled by color (green, carbon; blue, nitrogen; red, oxygen). Apostrophes after residue numbers indicate the position of the residue in an adjacent polypeptide chain. The figures are based on data for the CTP-liganded structure and the bisubstrate analogue PALA-liganded structure, respectively (35, 51).