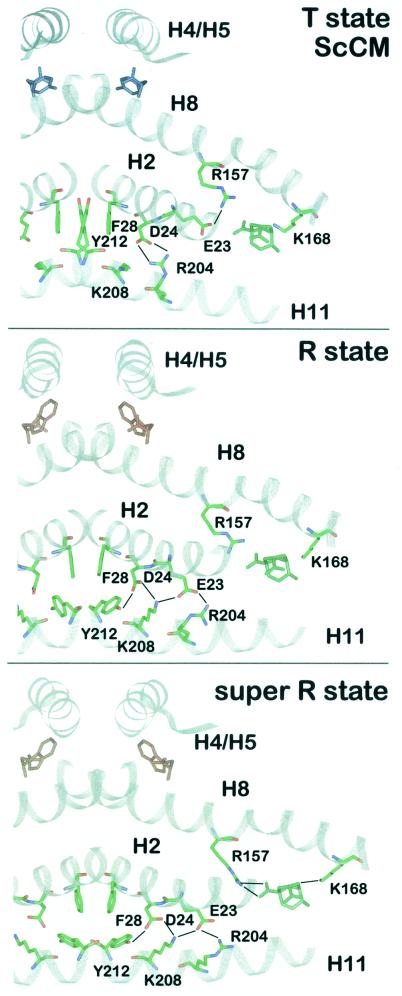
FIG. 6.
Intramolecular signaling pathway. A section of the ScCM dimer is presented in the T state (top), R state (middle), or super R state (bottom). The polypeptide backbone is drawn in ribbon style. The residues which change their position during allosteric transition and thereby transduce the signal of effector binding from the allosteric to the active site are shown as stick models (green, carbon; blue, nitrogen; red, oxygen). Hydrogen bonds are indicated by black lines. The position of the catalytic site inhibitor in the T and R state is derived from a superposition with the super R state structure using residues 1 to 214 and 224 to 254. Tyrosine is colored blue, tryptophan is red, and the bicyclic inhibitor is green.