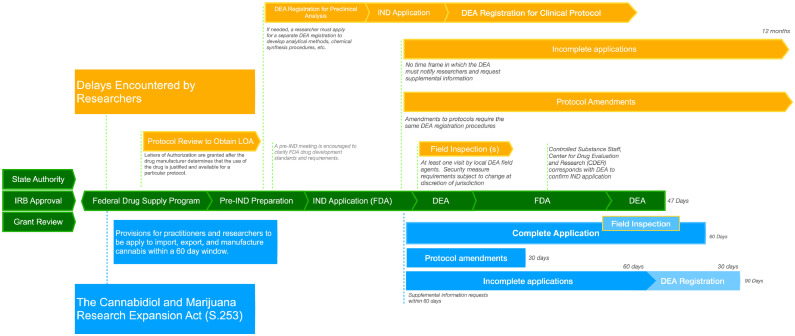
Figure 2.
Regulatory processes and DEA registration to conduct clinical research on cannabis in the US.
The total registration process is currently divided into a pre-registration (left) and a registration process (right). Prior to initiating a Schedule I research application with the DEA, investigators must obtain approval and documentation from their respective state authority and IRB. A researcher who seeks federal funds to conduct a study will often submit their protocol to be assessed by one of the science-focused federal institutions as part of a grant review.9 Protocols may also be reviewed by NIDA DSP or another federally approved manufacturer to obtain an LOA, which allows the FDA to reference detailed information on a particular cultivar, or strain, including chemistry, manufacturing, and controls during the review of an IND application. Obtaining drug product and a Letter of Authorization (LOA) has been a notable barrier for researchers as cannabis from NIDA DSP, the single federal source of cannabis before a recent DEA policy change, has been described as poor quality, of limited variety, and often available in limited or insufficient quantities. As of May 2022, an additional six manufacturers have been licensed to manufacture cannabis for research purposes. S.253 provides an avenue for new cannabis manufacturing registration applications to be processed in a timely manner, requiring the Attorney General to approve eligible new manufacturers within 60 days (blue). Regulatory hurdles and delays have been reported at every step of these processes (yellow), well before the DEA will consider an applicant.