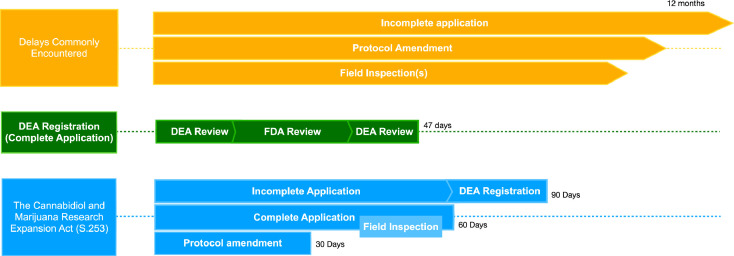
Figure 3.
DEA registration for interventional research with cannabis.
Only approximately 30% of researcher registration applications are complete at the time of submission. In this case, a registration would be issued within 47 days (green). However, the DEA reports an average processing time of 52 days once an application is complete.31 Typical delays (orange) are reported in the 6 – 12-month range. S.253 (blue) would mandate a total timeframe of no more than 60 days for complete applications. Any supplemental material must be requested by the DEA within 60 days. Upon receipt of a supplemental application, the DEA would be required to grant a registration or denial with a written explanation within 30 days. Protocol amendments are processed and assumed to be accepted within 30 days of receipt by the DEA unless they involve changes in drug quantity, source, or storage conditions. If any of the latter is required, the researcher may provide supplemental information detailing the necessary safeguards taken. In these circumstances, the Attorney General (AG) must provide written approval or instructions to modify the protocol accordingly within 10 days of receiving an amendment.