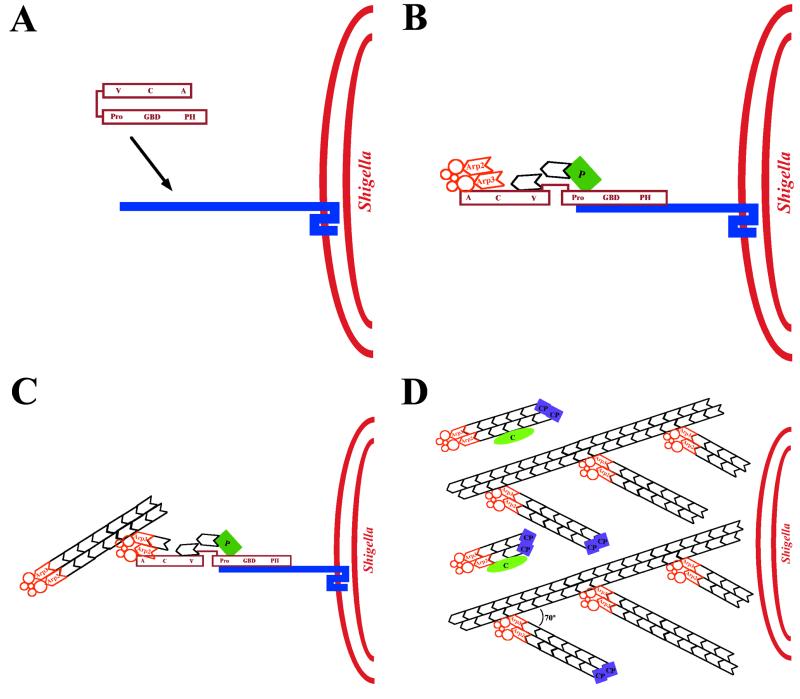
FIG. 20.
Model of actin tail assembly by Shigella IcsA. (A) IcsA (blue bar) on the surface of Shigella binds N-WASP (maroon). (B) IcsA binding disrupts the intramolecular bonds and activates N-WASP, thereby stimulating N-WASP activation of the Arp2/3 complex (orange), N-WASP binding to monomeric actin (black hexagon), and possibly N-WASP binding to profilin-actin (P, green rectangle). The Arp2/3 complex nucleates actin, mediates the addition of actin monomers to the barbed end, and caps the pointed end of a new actin filament. (C) As the filament extends, the original Arp2/3 complex is released from N-WASP and another Arp2/3 complex binds both N-WASP and the side of an existing actin filament. Each interaction stimulates the actin-nucleating activity of the Arp2/3 complex. (D) Repeated rounds of filament branching, filament nucleation, filament extension, and Arp2/3 complex release generate a network of actin filaments linked at 70° angles. At a distance from the bacterial surface, filament barbed ends are capped, thereby halting the addition of more actin monomers. Also at a distance from the bacterial surface, filaments debranch and, with the assistance of the actin-severing protein cofilin, depolymerize, thereby maintaining a local pool of actin monomers. Whether cofilin also severs filaments near the bacterial surface, thereby generating uncapped barbed ends, is unknown. V, verprolin homology domain; C, cofilin homology domain; A, acidic domain; Pro, proline-rich region; GBD, GTPase-binding domain; PH, pleckstrin homology domain.