Abstract
Background
Liver transplantation is a proven management method for end-stage cirrhosis and is estimated to have increased life expectancy by 15 years. The COVID-19 pandemic posed a challenge to patients who were candid for a solid-organ transplant. It has been suggested that the outcomes of liver transplants could be adversely affected by the infection, as immunosuppression makes liver transplant candidates more susceptible to adverse effects while predisposing them to higher thrombotic events.
Material and methods
In this retrospective study, the cases who received liver transplants from January 2018 to March 2022 were assessed regarding early postoperative mortality rate and hepatic artery thrombosis (HAT) with COVID-19 infection. This study included 614 cases, of which 48 patients were infected.
Results
This study shows that the early COVID-19-related early postoperative mortality rates substantially increased in the elective setting (OR: 2.697), but the results for the acute liver failure were insignificant. The average model for end-stage liver disease score increased significantly during the pandemic due to new regulations. Although mortality rates increased during the pandemic, the data for the vaccination period show that mortality rates have equalised with the prepandemic era. Meanwhile, COVID-19 infection is assumed to have increased HAT by 1.6 times in the elective setting.
Conclusion
This study shows that COVID-19 infection in an acute liver failure poses comparatively little risk; hence transplantation should be considered in such cases. Meanwhile, the hypercoagulative state induced by the infection predisposes this group of patients to higher HAT rates.
Keywords: cirrhosis, COVID-19, hepatic surgery
WHAT IS ALREADY KNOWN ON THIS TOPIC
Liver transplantation has in proven and effective method for the treatment of cirrhosis and acute liver failure, although with a relatively high mortality rate; model for end-stage liver disease and Child scores have been used to evaluate the risk of mortality. During the pandemic, the active disease was considered a contraindication for transplantation, but many of the patients did and still do get infected after the surgery, which is assumed to have increased both the mortality and hepatic artery thrombosis rate.
WHAT THIS STUDY ADDS
This study shows that post-transplantation infection with COVID-19 significantly increases mortality rates for elective surgeries; however, this result was not replicated for acute liver failure cases. Moreover, hepatic artery thrombosis increases with an infection which shows a direct relation between COVID-19 infection and thrombotic events.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
As mortality rates did not increase significantly with COVID-19 infection in patients with acute liver failure, it is suggested that these patients still undergo transplantation.
Introduction
COVID-19 pneumonia caused by the SARS-CoV-2 virus first emerged in Wuhan, China, and ever since the disease became a pandemic, many individuals have been affected by the disease. The highly virulent and contagious disease has affected all age groups and cases with different risk factors.1 It has been stated that a concurrent underlying disease such as hypertension, diabetes and age could significantly increase mortality and morbidity rates.1–3 one of the most prominent risk factors for severe COVID-19 infection is immunosuppression.1 2 4 5 Regardless of the aetiology for which cases are immune-compromised, the outcomes of infections can worsen. Liver transplantation is particularly affected by the infection as not only does immunosuppression predisposes cases to more severe consequences, but also the infection itself might worsen liver failure status.6–10
Hepatic artery thrombosis (HAT) is one of the major complications after liver transplantation, significantly increasing post-transplantation mortality rates.11 12 ever since the first studies of COVID-19 were carried out, it was realised that the infection increases coagulopathic incidences such as stroke.13 14 Even though several cases of post-transplantation HAT have been reported, the correlation between the two incidences have not been studied.15 16
During the COVID-19 era, many elective surgeries were postponed as hospital beds were needed for severely affected cases. Meanwhile, emergent surgery was still required to treat cases in an emergent state properly. Given that the current literature has not assessed and evaluated the outcomes of liver transplantation, this study was designed to assess the early postoperative mortality rate and HAT incidence rates before and after the COVID-19 pandemic in both elective and emergent settings.
Materials and methods
This study is a retrospective cross-sectional case–control study that assessed early postoperative mortality and transplantation outcomes of cases who underwent liver transplantation before and after the COVID-19 pandemic; thus, the subjects included all cases who were referred for transplantation from January 2018 to March 2022. The data of this study were extracted from the hepatobiliary and liver transplantation database of Imam hospital complex, affiliated with the Tehran University of medical sciences.
This study’s cases included all adult cases diagnosed with early postoperative COVID-19 infection, while the control group included the cases whose infection was ruled out. No paediatric cases were included in this study. According to the guidelines, If any patient was diagnosed with active pulmonary manifestations such as diffuse parenchymal involvement in chest CT scan or low Q2 saturation or any haemodynamic instability before surgical intervention, the surgical procedure was cancelled, but acute cases who were in dire need of transplantation, the procedure was carried out if the patient was stable with high oxygen saturation (>96%) with minimal pulmonary involvement (<5%). Each group was assessed in both elective and acute liver failures. COVID-19 infection was diagnosed using a PCR test and/or a CT scan in favour of infection (CT scans are reviewed by two expert specialists in infectious diseases and radiology). The cases’ demographics and data pertaining to the aetiology of liver failure are gathered. Patient risk assessment and the need for a transplant were carried out using Child-Pugh and MELD scores (model for end-stage liver disease). The cases in both groups were matched for age and comorbidities and compared with early postoperative mortality and HAT. In order to further assess the cases with respect to age strata, the cases were assessed in two age strata; older and younger than 60. It is also worth noting that since the number of elective cases which required transplantation during the pandemic was on the rise, along with the fact that many whole organ donations would be lost, and many cases who were on the waiting list for a long time were getting to more critical state. The Iranian committee of liver transplant decided that the elective cases with a MELD score higher than 25 could receive liver transplant during the peaks of the pandemic in selected centres (including our centre) with highest precautions, due to a large number of waiting list mortality of cirrhotic patient and availability of proper organ from brain death donors. In this group, liver transplantation was carried out only if the preoperative COVID-19 test was negative and the chest CT scan had nothing in favour of COVID-19 infection. With respect to donors, we only used DBD donations; all donors were screened for COVID-19 infection using PCR tests and CT scans. According to the national protocols, the majority of people were vaccinated in the last 6 months of the study with the Sinopharm BIBP COVID-19 vaccine, also known as BBIBP-CorV.
Analysis was carried out after the cases and controls were matched; as MELD and CHILD scores predict early postoperative mortality rates, we furthered analysis using adjusted regression models. Even though HAT is an incidental finding and a rare complication of transplantation itself, adjustments for age and other factors using regression models were made.
With respect to immunosuppression protocol in infected cases, the protocol for treatment was revised and assessed by a multidisciplinary team of physicians consisting of surgical, hepatobiliary and infectious diseases specialists. This protocol indicated that the cases received a lower single dose of intravenous pulse steroid (250 mg of methylprednisolone vs 1 g in routine situations as our centre protocol) followed by 5 days of corticosteroid (dexamethasone 8 mg) three times a day. Antimetabolites such as mycophenolate mofetil were not administered if pneumonia was present and were initiated if the patient was fully recovered with two negative COVID-19 PCR tests. Tacrolimus, as the main post-transplantation immunosuppressive drug, was administered 48 hours after surgery; the protocol was altered and tailored to each patient if they did not show signs of improvement.
Statistical analysis
Data analysis was performed by IBM’s SPSS software V.25. χ2 and Fisher’s exact tests were used to analyse the correlation. Logistic regression models were also used to determine the probability of outcomes. To determine the normality of data Kolmogorov-Smirnov test was used. In this study, cases and controls were matched for age, sex, CHILD and MELD scores. P values under 0.05% were considered significant.
Results
This study included 614 cases, out of which 48 cases were diagnosed with early COVID-19 infection. Of 48 cases who were diagnosed with the infection, 14 cases were acute cases and 34 cases were elective cases for transplantation. This study included 250 (40.7%) female and 364 (59.2%) male subjects. The cases were, on average, 42.82 years old (SD: 16.85). Mean MELD scores for cases with and without early COVID-19 infection were 25.47 (SD: 5.055) and 21.79 (SD: 5.67). Mean Child-Pugh scores for cases with and without early COVID-19 infection were 10.04 (SD: 1.42) and 9.89 (SD: 2.20), respectively. Due to new regulations that were implemented during the pandemic, the criteria for liver transplantation indicated that cases who were in a more critical state should receive transplantation; hence, while the mean average MELD score for elective cases changed significantly (t-test: 10.853, p=0.000) from the prepandemic mean of 19.52 (SD: 4.326) to the postpandemic mean score of 24.35 (SD:4.660), the mean score for acute liver failure changed from 25.000 (SD:8.752) to 29.360 (SD:7.034) although insignificantly (t-test: 1.928, p=0.060)
Regarding the early postoperative mortality rates, while mortality rates among COVID-19 positive cases in the elective setting are significantly higher, the rates among the cases of acute liver failure are statistically insignificant. This result was also applicable in adjusted regression models, which indicates that in elective settings, COVID-19 status plays a significant role and increases early postoperative mortality by more than two times (OR: 2.697) (table 1, figure 1).
Table 1.
Correlation status between early COVID-19 infection with mortality
| COVID-19 status | |||||
| Emergency status | Early COVID-19 status | Dead | Alive | χ2 and p value | Regression model (adjusted) |
| Elective | Negative | 110 (24.1%) | 346 (75.9%) | 8.714 (0.003) | Wald: 4.099 OR: 2.697 p value: 0.043 |
| Positive | 16 (47.1%) | 18 (52.9%) | |||
| Acute | Negative | 51 (46.4%) | 59 (53.6%) | 0.61 (0.804) | Wald: 0.173 OR: 1.595 p value: 0.67 |
| Positive | 6 (42.9%) | 8 (57.1%) | |||
Figure 1.
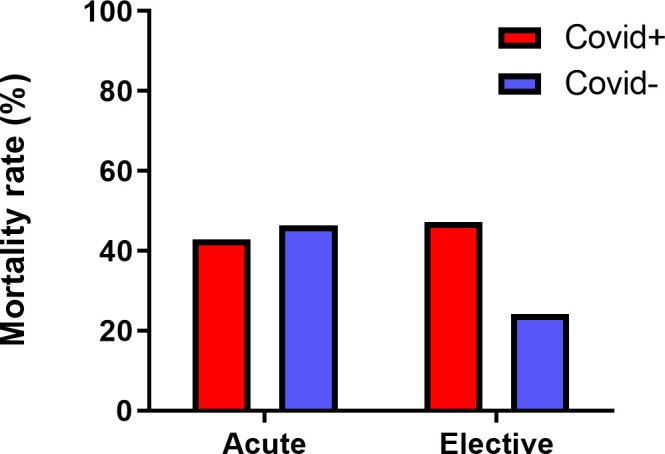
Total mortality rates with respect to COVID-19 infection status.
Regarding the primary or retransplantation status, the data show that retransplantation is significantly associated with early postoperative mortality. Due to limited data, reliable analysis for acute liver failure could not be carried out. During the pandemic and as the regulations changed and as new regulations in our country stated, elective transplantation in cases with infection was carried out only if they had a high MELD score; in this regard, we assessed early postoperative mortality rates before and after the start of the pandemic. Accordingly, while mortality rates among elective cases increased significantly, the rates were insignificant in acute liver failure (table 2, figure 2).
Table 2.
Correlation status between mortality rates in prepandemic or postpandemic eras in acute or elective settings
| Mortality rates in prepandemic and postpandemic eras | ||||
| Emergency status | Era | Dead | Alive | χ2 and p value (exact) |
| Elective | Prepandemic | 63 (21.9%) | 225 (78.1%) | 5.391 (0.021) OR: 1.61 |
| Postpandemic | 63 (31.2%) | 139 (68.8%) | ||
| Acute | Prepandemic | 34 (46.6%) | 39 (53.4%) | 0.026 (1.000) OR: 0.942 |
| Postpandemic | 23 (45.1%) | 28 (54.9%) | ||
Figure 2.
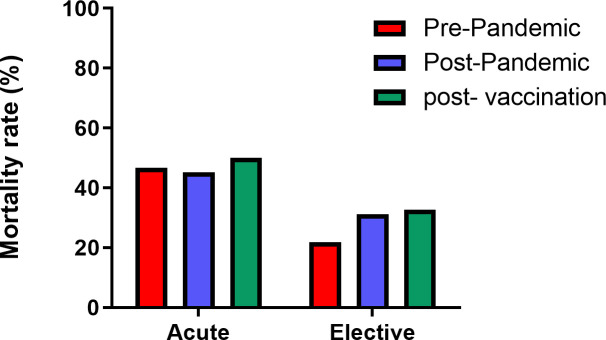
Comparison of mortality rates during the time before and after the start of the pandemic along with the rates pertaining to the last 6 months (postvaccination) leading to the end of the Persian calendar in which the majority of the population was vaccinated against COVID-19.
During the last 6 months to the end of the year in the Persian calendar (23 September 2021 to 20 March 2022) and as a nationwide vaccination programme was implemented, all cases were required to have full vaccination against COVID-19. Therefore, we assessed the data pertaining to this 6 month (postvaccination) with the prepandemic era. The results showed minor insignificant differences in mortality rates in elective and acute liver failures (table 3).
Table 3.
Correlation status between mortality rates in prevaccination or postvaccination eras in acute or elective settings
| Mortality rates in the prepandemic era and postvaccination | ||||
| Status | Dead | Alive | χ2 and p value (exact) | |
| Elective | Prepandemic | 63 (21.9%) | 225 (78.1%) | 2.711 (0.104) OR: 1.73 |
| Postvaccination | 16 (32.7%) | 33 (67.3%) | ||
| Acute | Prepandemic | 34 (46.6%) | 39 (53.4%) | 0.062 (1.000) OR: 1.14 |
| Postvaccination | 8 (50.0%) | 8 (50.0%) | ||
According to the literature, older age in recipients is a relative risk factor which increases mortality rates; in this study, we tried to assess whether the age of recipients significantly increases the mortality rates; therefore, we assessed the cases in two separate brackets of older and younger than 60. The statistical analysis shows that the age of recipients only affects the outcomes in acute, COVID-19-positive cases (p=0.024), however, Fisher’s exact test p=0.055, which showed insignificance (table 4).
Table 4.
The data presented in this table demonstrates and compares the mortality rates of cases older than 60 and younger in acute and elective settings
| Age strata and mortality | |||
| <60 | ≥60 | P value (Fisher’s exact test) | |
| Elective | 101 (24.8%) | 25 (30.5%) | 0.272 |
| Acute | 47 (43.1%) | 10 (66.7%) | 0.103 |
| Elective cases | |||
| COVID-19 positive | 14 (48.3%) | 2 (40.0%) | 0.732 |
| COVID-19 negative | 87 (23.0%) | 23 (29.9%) | 0.242 |
| Acute cases | |||
| COVID-19 positive | 3 (27.3%) | 3 (100%) | 0.055 |
| COVID-19 negative | 44 (44.9%) | 7 (58.3%) | 0.541 |
Regarding HAT, even though reliable statistical analysis for acute liver failure could not be carried out, the results for the elective setting show that with an OR of 4.02, COVID-19 infection increases risks significantly. However, this result was not replicated in regression models. The analysis regarding the changes during the pandemic shows a marked increase, although marginally statistically insignificant (table 5, figure 3).
Table 5.
The correlation between hepatic artery thrombosis (HAT) and COVID-19 infection status and era
| COVID-19 status | ||||
| Emergency status | Early COVID-19 status | HAT negative | HAT positive | χ2 and p value (exact) |
| Elective | Negative | 433 (95.2%) | 22 (4.8%) | 5.909 (0.032) OR: 4.02 |
| positive | 29 (85.3%) | 5 (14.7%) | ||
| Acute | Negative | 105 (96.3%) | 4 (3.7%) | 0.53 (1.000) |
| positive | 14 (100.0%) | 0 | ||
| Era status | ||||
| Emergency status | Era | HAT negative | HAT positive | χ2 and p value (exact) |
| Elective | Prepandemic | 276 (96.2%) | 11 (3.8%) | 3.798 (0.069) OR: 2.158 |
| Pandemic | 186 (92.1%) | 16 (7.9%) | ||
| Acute | Prepandemic | 70 (97.2%) | 2 (2.8%) | 0124 (1.000) OR:1.429 |
| Postpandemic | 49 (96.1%) | 2 (3.9%) | ||
| Total | Prepandemic | 346 (96.4%) | 13 (3.6%) | 3.767 (0.062) OR:2.039 |
| Postpandemic | 235 (92.9%) | 18 (7.1%) | ||
Figure 3.
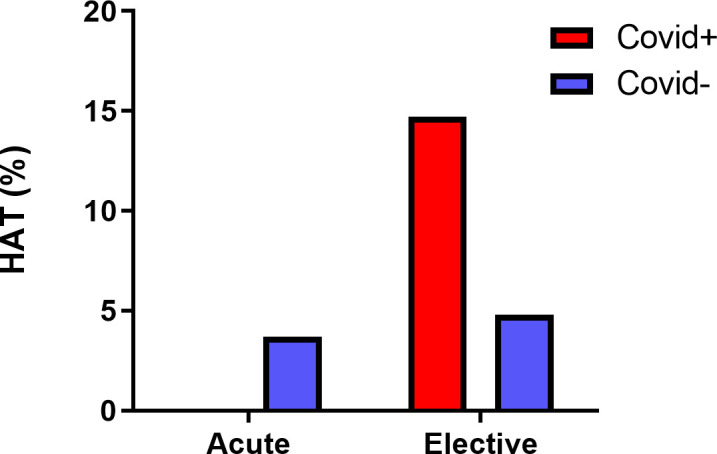
Hepatic artery thrombosis (HAT) rates with respect to COVID-19 infection. Note that the results for acute liver failure were insignificant, and more data might need to be more accurate due to a limited number of acute patients who develop HAT.
Discussion
This study shows that while COVID-19 infection significantly increases mortality rates among the cases in the elective setting, mortality rates do not significantly increase in acute liver failure. This might be due to the fact that liver failure, especially acute liver failure, poses a great risk; hence, transplantation is still mandatory in acute cases of COVID-19 infection. Meanwhile, the cases that require retransplantation are at a greater risk of mortality.
Regarding HAT, even though we could perform analysis in acute liver failure, we assume that COVID-19 increases thrombosis rates significantly in either setting. And the results show that the overall rate has increased during the pandemic, which might be due to thrombophilia that is subsequent to infection.
The COVID-19 pandemic has substantially affected medical practice, particularly in elective settings. Since the pandemic might last for many years to come, it is of utmost importance to assess the risks that cases might face if appropriate care is not provided. The currently available literature has not defined whether the acute cases that are affected by the disease still require urgent transplantation. This study has defined that in comparison to the risk that is posed by acute liver failure, COVID-19 infection poses a more negligible risk. Therefore, these cases still require urgent transplantation.
A multicentre survey conducted by Russo et al showed that liver transplantation policies significantly changed during the first wave of infection, and mortalities had increased significantly.6 A preliminary study in Italy showed that age-adjusted mortalities were higher than the general population.17 Meanwhile, a systematic review of case series by Gavriilidis and Pai showed that mortality rates were lower than the general population and the fatal cases mainly were over 65, but this interpretation could have been biased as it was based on case reports and series.18 A systematic review in 2020 states that immune-suppressed cases have higher diarrhoeal manifestation than the general population and older cases with risk factors such as dyspnoea and diabetes mellitus have a higher risk of mortality.19 An early systematic review in 2021 also states that higher mortality rates among older cases may be due to age-related risk factors.20 Our study is the first case–control study that assesses the early postoperative mortality rates among cases in both elective and acute liver failures. It provides a more comprehensive overview of the risks that are proposed solely by infection, as our analysis was adjusted for both Child-Pugh and MELD scores along with other risk factors.
Among various risk factors that determine the mortality rates of liver transplantation, hepatic encephalopathy and serum creatinine remain the most determinant factors; multiple studies have assessed the role of donor and recipient gender and age.21–24 In a study conducted by Evans et al,25 it has been stated that in patients older than 60 with a MELD score higher than 40, mortality rates increase significantly. Similarly, Gil et al26 state in a cohort study conducted in 2018 that mortality rates increase by four folds in patients older than 70. However, in another study conducted by Zetterman et al,27 it has been stated that the increased mortality rates are mostly age-related and not hepatic related. With respect to gender, it has widely been observed in multiple studies that older women have similar mortality and morbidity to that of older men, whereas younger women have better outcomes in comparison to male cases.21 22 28 This phenomenon is attributed chiefly to the liver protective effects of oestrogen, which sharply decreases with menopause.21 28 In this study, we adjusted the results to age, gender, MELD and CHILD scores in order to mitigate such effects. Moreover, we assessed the mortality rates in two age strata. This analysis showed that acute cases with concurrent infection might have higher mortality.
Regarding HAT, our study shows that COVID-19 significantly increases HAT chances HAT. The association between SARS-COV2 and thrombosis has been observed since the first cases were affected by the disease. Several case reports have shown that HAT can happen as a consequence of the infection.11 15 A recurrent form of HAT as a result of COVID-19 was reported in India, which shows the severity of HAT by which the cases might be affected.16
Our analysis was carried out after the cases and controls were matched; moreover, as MELD and CHILD scores predict mortality rates, we furthered research using adjusted regression models. These models yielded significant results for certain parts, indicating a significant correlation between early postoperative mortality rates and COVID-19 infection. in respect to HAT, we assume that even though HAT is a complication of transplantation itself, adjustments for age and other factors that are mentioned in the manuscript have shown that COVID-19 infection can, in fact, increase HAT rates.
Limitations
Due to retrospective and cross-sectional study quality, the results are limited. In specific fields of study, a limited number of cases might have led to insignificant results; therefore, with the current pandemic, more follow-up data are needed to assess the outcomes fully.
Conclusion
This study shows that while early postoperative mortality rates in acute liver failures might change only minimally, the mortality rates significantly increase among elective cases. Moreover, the chances of HAT increase considerably in cases affected by COVID-19.
Footnotes
Contributors: AM and AJ conceived the idea for the manuscript. AM, AH and FG collected data. AH and AM contributed to data analysis and interpretation. AH, AM, AJ, MNT and ZA drafted the manuscript. AJ and AM revised the manuscript and supervised the project. MA-T edited the manuscript grammatically. AH and SG prepared the tables and figures and blinded them to hide the cases’ personal information. AJ and AM stand as guarantors of the manuscript. All authors read and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request due to privacy/ethical restrictions.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study was approved by the Research Deputy and the Ethics Committee of the Tehran University of Medical Sciences (Reference number: IR.TUMS.MEDICINE.REC.1399.717) and conducted per the ethical standards laid down in the 1964 Declaration of Helsinki and all subsequent revisions. A written informed consent form was obtained from all the participants. Participants gave informed consent to participate in the study before taking part.
References
- 1.Sanyaolu A, Okorie C, Marinkovic A, et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med 2020;2:1069–76. 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rashedi J, Mahdavi Poor B, Asgharzadeh V, et al. Risk factors for COVID-19. Infez Med 2020;28:469–74. [PubMed] [Google Scholar]
- 3.Rod JE, Oviedo-Trespalacios O, Cortes-Ramirez J. A brief-review of the risk factors for covid-19 severity. Rev Saude Publica 2020;54:60. 10.11606/s1518-8787.2020054002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni AV, Tevethia HV, Premkumar M, et al. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. EClinicalMedicine 2021;38:101025. 10.1016/j.eclinm.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansoor E, Perez A, Abou-Saleh M, et al. Clinical characteristics, hospitalization, and mortality rates of coronavirus disease 2019 among liver transplant patients in the United States: a multicenter research network study. Gastroenterology 2021;160:459–462.S0016-5085(20)35214-8. 10.1053/j.gastro.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo FP, Izzy M, Rammohan A, et al. Global impact of the first wave of COVID-19 on liver transplant centers: a multi-society survey (EASL-ESOT/ELITA-ILTS). J Hepatol 2022;76:364–70:S0168-8278(21)02107-3. 10.1016/j.jhep.2021.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb GJ, Moon AM, Barnes E, et al. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol 2020;5:643–4:S2468-1253(20)30125-4. 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baganate F, Beal EW, Tumin D, et al. Early mortality after liver transplantation: defining the course and the cause. Surgery 2018;164:694–704:S0039-6060(18)30225-3. 10.1016/j.surg.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 9.Belli LS, Duvoux C, Karam V, et al. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol 2020;5:724–5:S2468-1253(20)30183-7. 10.1016/S2468-1253(20)30183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumortier J, Duvoux C, Roux O, et al. COVID-19 in liver transplant recipients: the French SOT COVID registry. Clin Res Hepatol Gastroenterol 2021;45:101639:S2210-7401(21)00018-8. 10.1016/j.clinre.2021.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stange BJ, Glanemann M, Nuessler NC, et al. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl 2003;9:612–20. 10.1053/jlts.2003.50098. [DOI] [PubMed] [Google Scholar]
- 12.Mourad MM, Liossis C, Gunson BK, et al. Etiology and management of hepatic artery thrombosis after adult liver transplantation. Liver Transpl 2014;20:713–23. 10.1002/lt.23874. [DOI] [PubMed] [Google Scholar]
- 13.Hanff TC, Mohareb AM, Giri J, et al. Thrombosis in COVID-19. Am J Hematol 2020;95:1578–89. 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McFadyen JD, Stevens H, Peter K. The emerging threat of (micro) thrombosis in COVID-19 and its therapeutic implications. Circ Res 2020;127:571–87. 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antunes de Brito CA, de Oliveira Filho JRB, Marques DT, et al. COVID-19 and hepatic artery thrombosis: a case report. Am J Case Rep 2021;22:e932531. 10.12659/AJCR.932531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raj A, Shankar V, Singhal S, et al. Recurrent hepatic artery thrombosis following living donor liver transplant as sequelae of SARS-cov-2 infection-a case report. SN Compr Clin Med 2021;3:2629–34. 10.1007/s42399-021-01076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrono D, Lupo F, Canta F, et al. Outcome of COVID-19 in liver transplant recipients: a preliminary report from northwestern Italy. Transpl Infect Dis 2020;22:e13353. 10.1111/tid.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavriilidis P, Pai M. The impact of COVID-19 global pandemic on morbidity and mortality of liver transplant recipients children and adults: a systematic review of case series. J Clin Med Res 2020;12:404–8. 10.14740/jocmr4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser J, Mousley J, Testro A, et al. Clinical presentation, treatment, and mortality rate in liver transplant recipients with coronavirus disease 2019: a systematic review and quantitative analysis. Transplant Proc 2020;52:2676–83:S0041-1345(20)32634-8. 10.1016/j.transproceed.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayant K, Reccia I, Virdis F, et al. COVID-19 in hospitalized liver transplant recipients: an early systematic review and meta-analysis. Clin Transplant 2021;35:e14246. 10.1111/ctr.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keswani RN, Ahmed A, Keeffe EB. Older age and liver transplantation: a review. Liver Transpl 2004;10:957–67. 10.1002/lt.20155. [DOI] [PubMed] [Google Scholar]
- 22.Jo YY, Choi YS, Joo DJ, et al. Pretransplant mortality predictors in living and deceased donor liver transplantation. J Chin Med Assoc 2014;77:16–20:S1726-4901(13)00246-3. 10.1016/j.jcma.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Durand F, Levitsky J, Cauchy F, et al. Age and liver transplantation. J Hepatol 2019;70:745–58:S0168-8278(18)32626-6. 10.1016/j.jhep.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Burra P, De Martin E, Gitto S, et al. Influence of age and gender before and after liver transplantation. Liver Transpl 2013;19:122–34. 10.1002/lt.23574. [DOI] [PubMed] [Google Scholar]
- 25.Evans MD, Diaz J, Adamusiak AM, et al. Predictors of survival after liver transplantation in patients with the highest acuity (MELD ≥40). Ann Surg 2020;272:458–66. 10.1097/SLA.0000000000004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gil E, Kim JM, Jeon K, et al. Recipient age and mortality after liver transplantation: a population-based cohort study. Transplantation 2018;102:2025–32:12. 10.1097/TP.0000000000002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zetterman RK, Belle SH, Hoofnagle JH, et al. Age and liver transplantation: a report of the liver transplantation database. Transplantation 1998;66:500–6. 10.1097/00007890-199808270-00015. [DOI] [PubMed] [Google Scholar]
- 28.Gong N, Jia C, Huang H, et al. Predictors of mortality during initial liver transplant hospitalization and investigation of causes of death. Ann Transplant 2020;25:e926020. 10.12659/AOT.926020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request due to privacy/ethical restrictions.