Abstract
Background
The significantly prognostic value of fibrinogen-to-albumin ratio (FAR) has been proved in patients with coronary artery disease and different oncologic disorders. This study aimed to investigate the predictive value of FAR for left ventricular systolic dysfunction (LVSD) in acute coronary syndromes (ACS) patients.
Methods
A total of 650 ACS patients after percutaneous coronary intervention (PCI) were eventually enrolled in the analysis. Participants were classified into three groups according to baseline FAR levels (T1: FAR < 73.00; T2: 73.00 ≤ FAR < 91.00; T3: FAR ≥ 91.00). The association between FAR and LVSD was assessed by binary logistic regression analysis. A nomogram to predict the risk of LVSD was constructed based on the output indices from multivariate regression analyses.
Results
Patients with LVSD showed significantly higher FAR, monocyte-to-lymphocyte ratio (MLR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) than those without. FAR was an independent predictor of left ventricular dysfunction from the multivariate analyses (OR, 1.038; 95%CI, 1.020–1.057; P < 0.001). The area under receiver operating characteristic curve (AUC) of FAR predicting the occurrence of LVSD was 0.735. Meanwhile, FAR was the most powerful predictor than MLR, NLR, and PLR. Nomogram with the AUC reaching 0.906 showed a robust discrimination.
Conclusions
Admission FAR is independently and significantly associated with LVSD in patients with ACS undergoing PCI.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-023-01029-2.
Keywords: Fibrinogen-to-albumin ratio, Left ventricular ejection fraction, Left ventricular systolic dysfunction, Inflammation, Acute coronary syndrome
Background
Acute coronary syndrome (ACS) remains the leading cause of morbidity and mortality worldwide despite prolonged and rigorous cardiovascular risk factor management [1]. Left ventricular systolic dysfunction (LVSD) is a common and serious complication of acute myocardial infarction (AMI), which can lead to greatly increased risks of sudden death and heart failure (HF) [2]. LVSD remains a major prognostic indicator for adverse cardiovascular events in patients with coronary artery disease (CAD) [3]. The presentation of left ventricular dysfunction shows a significant impact on the prognosis of ACS patients. Left ventricular ejection fraction (LVEF) is a conventional parameter to evaluate left ventricular systolic function in clinical practice and has been recognized as a significantly independent predictor of mortality in patients with ACS [4, 5]. In this context, the evaluation of clinical biomarkers associated with the occurrence of left ventricular dysfunction for further optimal management is considered to improve risk stratification in ACS patients.
Fibrinogen-to-albumin ratio (FAR) is measured by dividing serum fibrinogen by serum albumin. Both fibrinogen and albumin are reliable indicators of chronic systemic inflammation. Inflammation plays a crucial part in the initiation and progression of the atherosclerotic plaque rupture, thrombus formation and endothelial dysfunction [6]. Several studies have demonstrated that inflammatory biomarkers, including neutrophil-to-lymphocyte ratio (NLR) [7], platelet-to-lymphocyte ratio (PLR) [8], monocyte-to-lymphocyte ratio (MLR) [9], fibrinogen [10] and albumin [11], correlate with the prognosis of ACS. However, the predictive role of FAR in occurrence of left ventricular dysfunction in ACS patients is still indistinct. This study aims to explore the significance of FAR on the occurrence of LVSD, so as to provide insights for the role of inflammation in the deterioration of left ventricular function in patients with ACS. Moreover, We aim to compare the predictive value of FAR, NLR, PLR, and MLR for LVSD to provide instructions for clinical treatment of ACS patients.
Methods
Participants
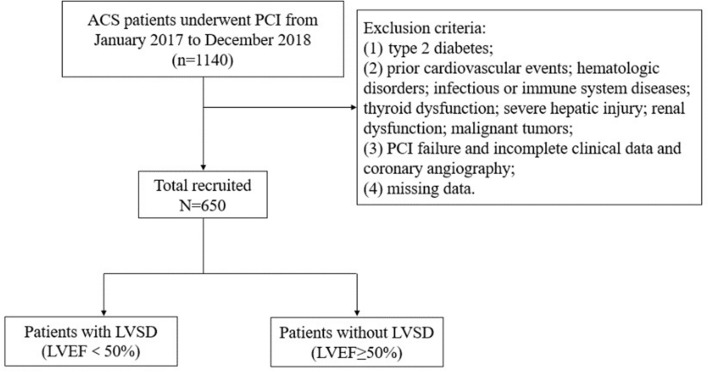
Patients who were diagnosed with ACS and underwent percutaneous coronary intervention (PCI) were consecutively enrolled from January 2017 and December 2018 at the First Affiliated Hospital of Medical College of Xi’an Jiaotong University in this single-center, retrospective, observational cohort study. The inclusion criteria were as follows: (1) age ≥ 18 years; (2) diagnosis of ACS, including unstable angina (UA), non-ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI); (3) treated with elective PCI. The exclusion criteria included patients with prior cardiovascular events; type 2 diabetes; severe hepatic injury; hematologic disorders; acute infection; immune system diseases; thyroid dysfunction; renal insufficiency or chronic dialysis; malignant tumors; pregnancy; PCI failure; incomplete clinical and angiographic data. Ultimately, a cohort of 650 patients based on strict inclusion and exclusion criteria were enrolled (Fig. 1). This retrospective study obtained the ethical approval from the Ethical Committee of the First Affiliated Hospital of Xi’an Jiaotong University and was performed in accordance with the principles of the Declaration of Helsinki.
Fig. 1.
Flowchart of the study population enrollment. ACS acute coronary syndromes, LVEF left ventricular ejection fraction, LVSD left ventricular systolic dysfunction, PCI percutaneous coronary intervention
Clinical data collection
Baseline data of demographic characteristics, including age, gender, weight, height, smoking, drinking, family history, and medication use were extracted from the standard medical records. BMI was calculated as weight in kilograms divided by squared height in meters (kg/m2). Heart rate and blood pressure measurements on admission was recorded. Patients with repeated systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, or receiving anti-hypertensive agents were considered criteria for hypertension [12]. Smoking was defined as an individual smoked a cigarette in the past 30 days or > 100 cigarettes in lifetime. Family history of CAD was defined as the occurrence of CAD in a first-degree relative. The routine hematology and biochemical parameters for baseline laboratory tests were drawn from the antecubital vein on admission and on the second day of hospitalization after an 8-h fast overnight. The Gensini score was calculated according to the results of coronary angiography. PCI was conducted in accordance with existing practice guidelines in China [13].
Definition of inflammatory markers
FAR is the ratio between serum fibrinogen and serum albumin. NLR is calculated by dividing neutrophil count by lymphocyte count. PLR is defined as the ratio of the platelet value and lymphocyte value. The ratio of the monocyte value and lymphocyte value means MLR.
Endpoint
LVEF was assessed using an ultrasonic cardiogram by two-dimensional Simpson’s method to determine the left ventricular systolic function. Patients were categorized into two groups based on their LVEF at 24 h after admission. Preserved systolic function was defined as LVEF ≥ 50% (n = 389) and LVSD was defined as LVEF < 50% (n = 261).
Statistical analysis
Continuous variables were, respectively, expressed as the mean ± standard deviation or median (interquartile ranges) according to whether normal distribution or not, while categorical variables were presented as percentages. The Kolmogorov–Smirnov test was used to analyze the normality of distribution. Student’s t test was used for comparison of continuous variables with normal distribution, and asymmetrically distributed variables were compared by Mann–Whitney U test, while percentages were analyzed by the Chi-squared test. The correlations between FAR and traditional cardiovascular risk factors were evaluated by adopting the Spearman’s rank correlation test or Pearson correlation test when variables appropriate. The receiver operating characteristic (ROC) curve was drawn to evaluate the diagnostic efficiency of inflammatory indicators for LVSD by determining the value of the area under the ROC curve (AUC) and the optimal cut-off values was counted according to the maximum Youden index. The predictive value of the FAR for LVEF was assessed by univariate and multivariate logistic regression model. Predictors of the endpoint determined by univariate analysis, potential confounders, and clinical importance were all included in multivariate analysis. Further subgroup analyses according to gender, age (< 65 and ≥ 65 years), hypertension, BMI (< 25 and ≥ 25 kg/m2), and diagnosis (NSTE-ACS and STEMI) were employed to examine the consistence of the prediction of FAR for LVSD. The performance of the nomogram was assessed by calibration and decision curve analyses (DCA). Statistical analyses were conducted using SPSS software version 23.0 and R 3.1.2. All the statistics are two-tailed and P < 0.05 was considered to be statistically significant.
Results
Patient characteristics
The baseline characteristics of enrolled patients stratified by the occurrence of LVSD (left ventricular systolic dysfunction) at admission are illustrated in Table 1. A total of 650 patients (age: 61.63 ± 10.57 years; 77.2% men) were finally enrolled in present study. Compared with those without LVSD, patients with systolic dysfunction had lower systolic blood pressure, higher heart rate and higher prevalence of smoking. Patients with LVSD presented higher level of NT-proBNP, white blood cells, NLR, MLR, PLR, hs-CRP, ALT, AST, creatinine, HCY, FAR, INR, APTT, FIB, d-dimer, FDP as well as higher prevalence of STEMI diagnosis but lower levels of albumin and apolipoprotein A. As for the angiographic findings, patients with LVSD were more likely to have three-vessel disease and significantly higher Gensini score. While there was no significant difference considering body mass index, age, drinking habits, hypercholesterolemia and FBG.
Table 1.
Baseline clinical and procedure characteristics of patients according to ejection fraction
| Baseline clinical characteristics | Total population (n = 650) | LVEF < 50% (n = 261) | LVEF ≥ 50% (n = 389) | P value |
|---|---|---|---|---|
| Age, years | 61.63 ± 10.57 | 62.08 ± 10.66 | 61.32 ± 10.51 | 0.418 |
| Sex, male, n (%) | 502 (77.2) | 217 (83.1) | 285 (73.3) | 0.003 |
| BMI, kg/m2 | 25.28 ± 3.19 | 25.19 ± 3.35 | 25.35 ± 3.04 | 0.427 |
| Heart rate, bpm | 74 (66–83) | 78 (68–89) | 72 (66–80) | < 0.001 |
| SBP, mmHg | 129.93 ± 19.75 | 125.21 ± 20.77 | 133.10 ± 18.39 | < 0.001 |
| DBP, mmHg | 79.79 ± 13.18 | 79.96 ± 14.58 | 79.68 ± 12.17 | 0.940 |
| Smoking, n (%) | 350 (53.8) | 166 (63.6) | 184 (47.3) | < 0.001 |
| Drinking, n (%) | 126 (19.4) | 47 (18.0) | 79 (20.3) | 0.467 |
| Hypertension, n (%) | 359 (55.2) | 121 (46.4) | 238 (61.2) | < 0.001 |
| Family history of CAD, n (%) | 71 (10.9) | 22 (8.4) | 49 (12.6) | 0.095 |
| NT-proBNP, pg/mL | 281.2 (92.2–1069.0) | 1075.5 (351.0–2840.0) | 132.5 (56.14–367.05) | < 0.001 |
| Cardiac troponin T, ng/mL | 0.305 (0.009–0.492) | 0.418 (0.044–1.575) | 0.110 (0.007–0.049) | < 0.001 |
| Hemoglobin, g/L | 141.48 ± 16.46 | 141.19 ± 17.32 | 141.68 ± 15.88 | 0.778 |
| Platelet, 109/L | 205.38 ± 63.37 | 206.43 ± 67.71 | 204.68 ± 60.36 | 0.851 |
| White blood cells, 109/L | 7.15 (5.61–9.47) | 8.40 (6.38–10.96) | 6.57 (5.32–8.35) | < 0.001 |
| Neutrophils, 109/L | 4.97 (3.66–7.09) | 6.55 (4.34–8.86) | 4.43 (3.40–5.87) | < 0.001 |
| Lymphocyte, 109/L | 1.43 (1.07–1.86) | 1.31 (0.98–1.79) | 1.52 (1.15–1.91) | < 0.001 |
| Monocytes, 109/L | 0.35 (0.28–0.47) | 0.41 (0.30–0.56) | 0.33 (0.26–0.43) | < 0.001 |
| NLR | 3.29 (2.31–5.71) | 4.79 (2.84–7.50) | 2.88 (2.06–4.05) | < 0.001 |
| MLR | 0.24 (0.18–0.34) | 0.28 (0.22–0.42) | 0.21 (0.16–0.29) | < 0.001 |
| PLR | 136.47 (104.06–185.04) | 149.31 (110.22–203.71) | 129.82 (97.81–169.92) | < 0.001 |
| hs-CRP, mg/L | 1.90 (0.77–5.40) | 3.59 (1.54–10.00) | 1.145 (0.54–3.07) | < 0.001 |
| ALT, U/L | 27 (18–40) | 30 (21–47) | 23 (16–35) | < 0.001 |
| AST, U/L | 26 (20–56) | 47 (26–116) | 22 (18–31) | < 0.001 |
| Albumin, g/L | 40.54 ± 4.65 | 38.86 ± 4.88 | 41.67 ± 4.13 | < 0.001 |
| BUN, mmol/L | 5.66 ± 1.78 | 5.77 ± 2.09 | 5.58 ± 1.54 | 0.992 |
| Scr, µmol/L | 67.78 ± 19.59 | 70.35 ± 23.19 | 66.05 ± 16.54 | 0.047 |
| Cystatin C, mg/L | 1.032 ± 0.326 | 1.079 ± 0.336 | 1.001 ± 0.316 | 0.005 |
| FPG, mg/dL | 4.74 (4.20–5.42) | 4.70 (4.27–5.44) | 4.77 (4.20–5.41) | 0.966 |
| RBG, mg/dL | 6.23 (5.31–7.68) | 6.23 (5.33–7.65) | 6.23 (5.29–7.71) | 0.776 |
| eGFR, mL/(min*1.73 m2) | 96.87 (88.69–104.62) | 95.77 (85.58–104.05) | 97.62 (90.35–105.52) | 0.058 |
| K+, mmol/L | 3.93 ± 0.39 | 3.92 ± 0.39 | 3.93 ± 0.40 | 0.629 |
| Na+, mmol/L | 141.04 ± 3.39 | 140.24 ± 3.92 | 141.57 ± 2.87 | < 0.001 |
| Ca2+, mmol/L | 2.30 ± 0.14 | 2.25 ± 0.14 | 2.33 ± 0.13 | < 0.001 |
| Uric acid, µmol/L | 340.25 ± 86.98 | 339.28 ± 91.75 | 340.91 ± 83.73 | 0.763 |
| Homocysteine, µmol/L | 17.7 (13.9–23.5) | 20.7 (15.8–33.4) | 15.9 (13.3–20.4) | < 0.001 |
| PT, s | 13.4 (13.0–13.8) | 13.7 (13.2–14.1) | 13.3 (12.9–13.7) | < 0.001 |
| PTA, % | 90.94 ± 13.64 | 87.83 ± 13.62 | 93.03 ± 13.27 | < 0.001 |
| INR | 1.04 (1.00–1.08) | 1.06 (1.02–1.11) | 1.03 (0.99–1.07) | < 0.001 |
| APTT, s | 36.4 (33.9–39.4) | 37.7 (34.7–41.4) | 35.9 (33.4–38.3) | < 0.001 |
| TT, s | 16.6 (15.8–17.4) | 16.6 (15.7–17.5) | 16.5 (15.9–17.3) | 0.727 |
| FIB, g/L | 3.33 (2.84–3.79) | 3.58 (3.12–4.32) | 3.15 (2.69–3.53) | < 0.001 |
| d-dimer, mg/L | 0.44 (0.30–0.70) | 0.56 (0.40–0.90) | 0.40 (0.30–0.56) | < 0.001 |
| FDP, mg/L | 1.20 (0.90–1.70) | 1.40 (0.96–2.30) | 1.20 (0.90–1.50) | < 0.001 |
| Triglycerides, mmol/L | 1.29 (0.97–1.82) | 1.17 (0.83–1.66) | 1.38 (1.04–1.97) | < 0.001 |
| TC, mmol/L | 3.74 (3.14–4.42) | 3.77 (3.12–4.46) | 3.73 (3.15–4.38) | 0.638 |
| LDL, mmol/L | 2.21 (1.68–2.79) | 2.28 (1.71–2.79) | 2.15 (1.65–2.79) | 0.242 |
| HDL, mmol/L | 0.91 (0.78–1.07) | 0.92 (0.78–1.06) | 0.91 (0.77–1.08) | 0.884 |
| apoA, g/L | 1.082 (0.967–1.212) | 1.067 (0.918–1.194) | 1.100 (0.995–1.232) | 0.001 |
| apoB, g/L | 0.763 (0.627–0.924) | 0.800 (0.627–0.934) | 0.751 (0.622–0.909) | 0.202 |
| apoE, g/L | 33.1 (26.5–40.8) | 32.5 (26.7–41.1) | 33.4 (26.3–40.7) | 0.877 |
| Lp (a), mg/L | 184 (95–338) | 239 (118–371) | 153 (86–309) | < 0.001 |
| LVEF, % | 60 (45–67) | 43 (39–47) | 66 (62–70) | < 0.001 |
| FAR | 81.43 (67.66–97.62) | 91.37 (79.38–116.50) | 75.14 (63.76–87.13) | < 0.001 |
| Gensini score | 62 (40–90) | 80 (50–100) | 52 (34–80) | < 0.001 |
| Initial diagnosis, n (%) | 0.138 | |||
| UA | 352 (54.2) | 59 (22.6) | 293 (75.3) | < 0.001 |
| NSTEMI | 82 (12.6) | 41 (15.7) | 41 (10.5) | 0.052 |
| STEMI | 216 (33.2) | 161 (61.7) | 55 (14.1) | < 0.001 |
| Killip class | ||||
| I | 285 (43.8) | 165 (63.2) | 120 (30.8) | < 0.001 |
| II | 303 (46.6) | 57 (21.8) | 246 (63.2) | < 0.001 |
| ≥ III | 62 (9.5) | 39 (14.9) | 23 (5.9) | < 0.001 |
| Diseased vessels number, n (%) | ||||
| One-vessel disease | 146 (12.9) | 142 (13.8) | 4 (3.9) | 0.005 |
| Two-vessel disease | 316 (28.0) | 294 (28.6) | 22 (21.6) | 0.131 |
| Three-vessel disease | 663 (58.7) | 587 (57.1) | 76 (74.5) | < 0.001 |
| Diseased vessels type, n (%) | ||||
| LM | 85 (7.5) | 76 (7.4) | 9 (8.8) | 0.601 |
| LAD | 1055 (93.4) | 958 (93.2) | 97 (95.1) | 0.460 |
| LCX | 845 (74.8) | 770 (74.9) | 75 (73.5) | 0.761 |
| RCA | 860 (76.1) | 779 (75.8) | 81 (79.4) | 0.412 |
| Target vessel territory, n (%) | ||||
| LAD | 742 (65.7) | 677 (65.9) | 65 (63.7) | 0.666 |
| LCX | 356 (31.5) | 325 (31.6) | 31 (30.4) | 0.800 |
| RCA | 482 (42.7) | 444 (43.2) | 38 (37.3) | 0.248 |
| Number of stents, n (%) | ||||
| 1 | 404 (35.8) | 365 (35.5) | 39 (38.2) | 0.583 |
| 2 | 341 (30.2) | 320 (31.1) | 21 (20.6) | 0.027 |
| ≥ 3 | 385 (34.1) | 343 (33.4) | 42 (41.2) | 0.112 |
| Average length of stents, mm | 26.79 ± 5.86 | 26.73 ± 5.84 | 27.39 ± 6.08 | 0.321 |
| Average width of stents, mm | 2.98 ± 0.43 | 2.98 ± 0.42 | 2.98 ± 0.43 | 0.652 |
| Plaque property, n (%) | ||||
| Calcification lesions | 142 (21.8) | 45 (17.2) | 97 (24.9) | 0.020 |
| Diffuse lesions | 171 (26.3) | 56 (21.5) | 115 (29.6) | 0.021 |
| Thrombus | 30 (4.6) | 18 (6.9) | 12 (3.1) | 0.023 |
| Chronic total occlusions | 93 (14.3) | 51 (19.5) | 42 (10.8) | 0.002 |
Data are presented as the IQR, mean ± SD or n (%)
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, CAD coronary artery disease, hs-CRP high-sensitivity C-reactive protein, NT-proBNP N-terminal pro-B type natriuretic peptide, ALT alanine transaminase, AST aspartate aminotransferase, BUN blood urea nitrogen, SCr serum creatinine concentration, FPG fasting plasma glucose, RBG random blood sugar, HbA1c glycosylated hemoglobin A1c, eGFR estimated glomerular filtration rate, K+ serum potassium, Na+ serum sodium, Ca2+ serum calcium, PT prothrombin time, PTA prothrombin time activity, INR international normalized ratio, APTT activated partial thromboplastin time, TT thrombin time, FIB fibrinogen, FDP fibrinogen degradation products, TC total cholesterol, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, apoA apolipoprotein A, apoB apolipoprotein B, apoE apolipoprotein E, Lp(a) Lipoprotein(a), LVEF left ventricular ejection fraction, UA unstable angina, NSTEMI non-ST-segment elevation myocardial infarction, STEMI ST-segment elevation myocardial infarction, LM left main artery, LAD left anterior descending artery, LCX left circumflex artery, RCA right coronary artery
Baseline clinical and procedure characteristics of patients categorized by the FAR tertiles are presented in Table 2. Patients with high FAR seemed to be older and higher heart rate. Laboratory indexes including NT-proBNP, cardiac troponin T, white blood cells, NLR, MLR, PLR, hs-CRP, cystatin C, FIB, FDP and FAR increased, whereas SBP, DBP, hemoglobin, triglycerides and LVEF decreased in proportion to the FAR tertiles. The rate of smoking, drinking, hypertension, family history of CAD and BMI level were not different among the different FAR groups. In the top FAR tertile, most patients were diagnosed as STEMI and showed significantly higher Gensini score.
Table 2.
Baseline clinical and procedure characteristics of patients stratified by the FAR tertiles
| Baseline clinical characteristics | T1 (n = 216) | T2 (n = 219) | T3 (n = 215) | P value |
|---|---|---|---|---|
| Age, years | 59.54 ± 10.88 | 62.32 ± 9.59 | 63.01 ± 10.93 | 0.001 |
| Sex, male, n (%) | 174 (80.6) | 169 (77.2) | 159 (74.0) | 0.263 |
| BMI, kg/m2 | 25.46 ± 3.08 | 25.20 ± 3.35 | 25.19 ± 3.13 | 0.558 |
| Heart rate, bpm | 72 (64–81) | 74 (66–82) | 76 (68–86) | 0.003 |
| SBP, mmHg | 132.2 ± 17.9 | 131.0 ± 20.7 | 126.5 ± 20.0 | 0.002 |
| DBP, mmHg | 81.3 ± 12.6 | 80.3 ± 13.2 | 77.7 ± 13.4 | 0.012 |
| Smoking, n (%) | 110 (50.9) | 118 (53.9) | 122(56.7) | 0.480 |
| Drinking, n (%) | 39 (18.1) | 50 (22.8) | 37(17.2) | 0.278 |
| Hypertension, n (%) | 124 (57.4) | 117 (53.4) | 118(54.9) | 0.700 |
| Family history of CAD, n (%) | 27 (12.5) | 23 (10.5) | 21(9.8) | 0.642 |
| NT-proBNP, pg/mL | 109.00 (53.53–286.40) | 263.25 (95.99–756.55) | 1020.00(297.85–3054.00) | < 0.001 |
| Cardiac troponin T, ng/mL | 0.012 (0.007–0.056) | 0.040 (0.009–0.402) | 0.198(0.015–1.485) | < 0.001 |
| Hemoglobin, g/L | 144.87 ± 14.80 | 143.41 ± 15.66 | 136.12 ± 17.54 | < 0.001 |
| Platelet, 109/L | 199.80 ± 61.37 | 198.35 ± 49.99 | 218.16 ± 74.84 | 0.016 |
| White blood cells, 109/L | 6.89 (5.36–9.08) | 6.81 (5.50–9.40) | 7.59(5.98–9.94) | 0.012 |
| Neutrophils, 109/L | 4.82 (3.46–6.60) | 4.86 (3.56–7.00) | 5.47(4.09–7.57) | 0.008 |
| Lymphocyte, 109/L | 1.44 (1.07–1.86) | 1.43 (1.11–1.85) | 1.45(1.04–1.87) | 0.853 |
| Monocytes, 109/L | 0.32 (0.25–0.41) | 0.34 (0.28–0.44) | 0.42(0.31–0.57) | < 0.001 |
| NLR | 3.10 (2.17–4.60) | 3.28 (2.25–5.93) | 3.61(2.51–6.23) | 0.029 |
| MLR | 0.22 (0.16–0.28) | 0.24 (0.18–0.32) | 0.29(0.19–0.43) | < 0.001 |
| PLR | 131.37 (101.45–171.25) | 134.61 (99.48–179.41) | 140.35(106.66–203.29) | 0.040 |
| hs-CRP, mg/L | 0.78 (0.39–1.85) | 1.77 (0.90–3.93) | 5.77(2.86–10.00) | < 0.001 |
| ALT, U/L | 25 (18–39) | 27 (18–38) | 26(18–44) | 0.001 |
| AST, U/L | 23 (19–33) | 26 (20–57) | 31(20–73) | 0.777 |
| Albumin, g/L | 43.44 ± 3.94 | 40.85 ± 3.89 | 37.31 ± 3.94 | < 0.001 |
| BUN, mmol/L | 5.69 ± 1.52 | 5.52 ± 1.59 | 5.77 ± 2.18 | 0.390 |
| Scr, µmol/L | 67.19 ± 16.91 | 66.10 ± 17.21 | 70.08 ± 23.78 | 0.290 |
| Cystatin C, mg/L | 1.006 ± 0.318 | 1.012 ± 0.331 | 1.079 ± 0.326 | 0.033 |
| FPG, mg/dL | 4.64 (4.16–5.34) | 4.85 (4.27–5.52) | 4.74(4.18–5.37) | 0.176 |
| RBG, mg/dL | 6.30 (5.31–7.84) | 6.35 (5.45–7.73) | 6.11(5.19–7.37) | 0.131 |
| eGFR, mL/(min*1.73 m2) | 98.18 (90.43–106.43) | 98.31 (90.76–104.44) | 94.27(84.78–102.40) | 0.001 |
| K+, mmol/L | 3.92 ± 0.37 | 3.92 ± 0.39 | 3.94 ± 0.42 | 0.884 |
| Na+, mmol/L | 141.33 ± 2.89 | 141.16 ± 3.01 | 140.62 ± 4.13 | 0.281 |
| Ca2+, mmol/L | 2.34 ± 0.12 | 2.31 ± 0.15 | 2.25 ± 0.14 | < 0.001 |
| Uric acid, µmol/L | 347.16 ± 88.77 | 342.33 ± 87.12 | 331.18 ± 84.60 | 0.148 |
| Homocysteine, µmol/L | 16.4 (13.7–22.8) | 17.9 (13.6–23.7) | 18.0 (14.6–24.5) | 0.224 |
| PT, s | 13.3 (12.9–13.7) | 13.4 (13.0–13.8) | 13.6 (13.2–14.1) | < 0.001 |
| PTA, % | 93.71 ± 12.73 | 92.07 ± 13.69 | 87.03 ± 13.63 | < 0.001 |
| INR | 1.03 (0.99–1.07) | 1.03 (0.99–1.07) | 1.06 (1.02–1.11) | < 0.001 |
| APTT, s | 35.95 (33.1–38.6) | 36.2 (33.9–39.4) | 37.0 (34.4–40.7) | 0.009 |
| TT, s | 16.8 (16.1–17.5) | 16.6 (15.9–17.4) | 16.1 (15.4–17.1) | < 0.001 |
| FIB, g/L | 2.66 (2.44–2.92) | 3.33 (3.09–3.51) | 4.10 (3.69–4.77) | < 0.001 |
| d-dimer, mg/L | 0.40 (0.30–0.50) | 0.41 (0.30–0.60) | 0.60 (0.40–1.10) | < 0.001 |
| FDP, mg/L | 1.00 (0.70–1.30) | 1.20 (0.91–1.50) | 1.60 (1.20–2.70) | < 0.001 |
| Triglycerides, mmol/L | 1.37 (1.09–1.92) | 1.30 (0.99–1.88) | 1.17 (0.85–1.63) | < 0.001 |
| TC, mmol/L | 3.80 (3.19–4.52) | 3.73 (3.15–4.46) | 3.59 (3.07–4.29) | 0.223 |
| LDL, mmol/L | 2.28 (1.70–2.87) | 2.22 (1.68–2.79) | 2.05 (1.65–2.72) | 0.176 |
| HDL, mmol/L | 0.93 (0.79–1.09) | 0.91 (0.77–1.07) | 0.90 (0.76–1.02) | 0.138 |
| apoA, g/L | 1.136 (1.025–1.240) | 1.083 (0.984–1.228) | 1.035 (0.903–1.162) | < 0.001 |
| apoB, g/L | 0.770 (0.630–0.922) | 0.751 (0.629–0.928) | 0.757 (0.617–0.910) | 0.907 |
| apoE, g/L | 33.9 (26.0–41.2) | 32.5 (26.6–42.0) | 32.5 (26.7–39.4) | 0.703 |
| Lp (a), mg/L | 148 (88–301) | 171 (84–333) | 236 (121–378) | 0.001 |
| LVEF, % | 64 (55–69) | 62 (46–69) | 47 (42–63) | < 0.001 |
| FAR | 62.80 (57.57–67.66) | 81.46 (77.29–85.28) | 107.14 (97.65–127.86) | < 0.001 |
| Gensini score | 52 (34–84) | 62 (40–88) | 72 (48–98) | 0.001 |
| Initial diagnosis, n (%) | ||||
| UA | 147 (68.1) | 122 (55.7) | 83 (38.6) | < 0.001 |
| NSTEMI | 16 (7.4) | 32 (14.6) | 34 (15.8) | 0.017 |
| STEMI | 53 (24.5) | 65 (29.7) | 98 (45.6) | < 0.001 |
| Killip class, n (%) | ||||
| I | 78 (36.1) | 101 (46.1) | 106 (49.3) | 0.016 |
| II | 123 (56.9) | 102 (46.6) | 78 (36.3) | < 0.001 |
| ≥ III | 15 (6.9) | 16 (7.3) | 31 (14.4) | 0.012 |
| Diseased vessels number, n (%) | ||||
| One-vessel disease | 44 (20.4) | 51 (23.3) | 44 (20.5) | 0.700 |
| Two-vessel disease | 69 (31.9) | 49 (22.4) | 49 (22.8) | 0.036 |
| Three-vessel disease | 103 (47.7) | 119 (54.3) | 122 (56.7) | 0.148 |
| Diseased vessels type, n (%) | ||||
| LM | 16 (7.4) | 21(9.6) | 21 (9.8) | 0.632 |
| LAD | 202 (93.5) | 199 (90.9) | 208 (96.7) | 0.042 |
| LCX | 142 (65.7) | 144 (65.8) | 145 (67.4) | 0.912 |
| RCA | 147 (68.1) | 161 (73.5) | 152 (70.7) | 0.457 |
| Target vessel territory, n (%) | ||||
| LAD | 130 (60.2) | 147 (67.1) | 141 (65.6) | 0.285 |
| LCX | 61 (28.2) | 76 (34.7) | 66 (30.7) | 0.340 |
| RCA | 113 (52.3) | 103 (47.0) | 98 (45.6) | 0.338 |
| Number of stents, n (%) | ||||
| 1 | 90 (41.7) | 88 (40.2) | 75 (34.9) | 0.316 |
| 2 | 66 (30.6) | 64 (29.2) | 76 (35.3) | 0.355 |
| ≥ 3 | 60 (27.8) | 67 (30.6) | 64 (29.8) | 0.803 |
| Average length of stents, mm | 27.61 ± 6.29 | 28.60 ± 5.83 | 27.47 ± 6.17 | 0.053 |
| Average width of stents, mm | 3.04 ± 0.45 | 2.99 ± 0.39 | 2.91 ± 0.44 | 0.016 |
| Plaque property, n (%) | ||||
| Calcification lesions | 47 (21.8) | 52 (23.7) | 43 (20.0) | 0.640 |
| Diffuse lesions | 56 (25.9) | 60 (27.4) | 55 (25.6) | 0.901 |
| Thrombus | 8 (3.7) | 13 (5.9) | 9 (4.2) | 0.505 |
| Chronic total occlusions | 25 (11.6) | 31 (14.2) | 37 (17.2) | 0.247 |
Data are presented as the IQR, mean ± SD or n (%)
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, CAD coronary artery disease, hs-CRP high-sensitivity C-reactive protein, NT-proBNP N-terminal pro-B type natriuretic peptide, FAR fibrinogen-to-albumin ratio, MLR monocyte-to-lymphocyte ratio, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, ALT alanine transaminase, AST aspartate aminotransferase, BUN blood urea nitrogen, SCr serum creatinine concentration, FPG fasting plasma glucose, RBG random blood sugar, HbA1c glycosylated hemoglobin A1c, eGFR estimated glomerular filtration rate, K+ serum potassium, Na+ serum sodium, Ca2+ serum calcium, PT prothrombin time, PTA prothrombin time activity, INR international normalized ratio, APTT activated partial thromboplastin time, TT thrombin time, FIB fibrinogen, FDP fibrinogen degradation products, TC total cholesterol, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, apoA apolipoprotein A, apoB apolipoprotein B, apoE apolipoprotein E, Lp(a) Lipoprotein(a), LVEF left ventricular ejection fraction, UA unstable angina, NSTEMI non-ST-segment elevation myocardial infarction, STEMI ST-segment elevation myocardial infarction, LM left main artery, LAD left anterior descending artery, LCX left circumflex artery, RCA right coronary artery
Correlation between FAR with LVEF and other cardiovascular risk factors
Spearman correlation analysis revealed significantly negative associations between LVEF and FAR (r = − 0.360, P < 0.001) (Fig. 2). FAR was positively correlated with age, HR, NT-proBNP, cardiac troponin T, platelet, white blood cells, neutrophils, monocytes, NLR, MLR, PLR, hs-CRP, AST, cystatin C, PT, INR, APTT, D-dimer, FDP and Lp (a), while negatively correlated with SBP, DBP, eGFR, serum Na+, serum Ca2+, PTA, TT, triglycerides, HDL, apoA (Table 3).
Fig. 2.
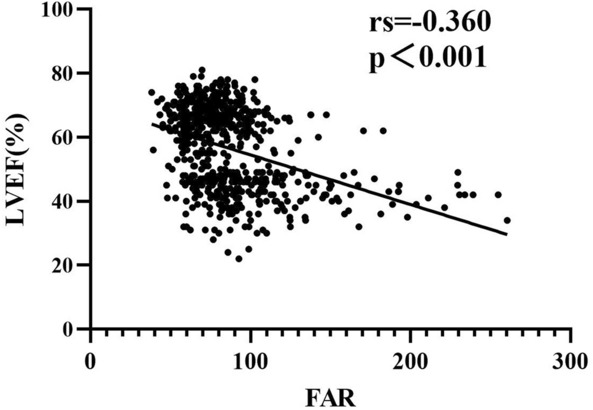
Scatter dot presentation comparison of FAR and LVEF. LVEF left ventricular ejection fraction, FAR fibrinogen-to-albumin ratio
Table 3.
Correlations between FAR and traditional cardiovascular risk factors
| Correlation coefficient | P value | |
|---|---|---|
| Age | 0.152 | < 0.001 |
| Heart rate | 0.152 | < 0.001 |
| SBP | − 0.143 | < 0.001 |
| DBP | − 0.120 | 0.002 |
| NT-proBNP | 0.514 | < 0.001 |
| Cardiac troponin T | 0.382 | < 0.001 |
| Platelet | 0.121 | 0.002 |
| White blood cells | 0.151 | < 0.001 |
| Neutrophils | 0.160 | < 0.001 |
| Monocytes | 0.274 | < 0.001 |
| NLR | 0.138 | < 0.001 |
| MLR | 0.259 | < 0.001 |
| PLR | 0.113 | 0.004 |
| hs-CRP | 0.590 | < 0.001 |
| AST | 0.184 | < 0.001 |
| Albumin | − 0.584 | < 0.001 |
| Cystatin C | 0.105 | 0.008 |
| eGFR | − 0.158 | < 0.001 |
| Na+ | − 0.100 | 0.011 |
| Ca2+ | − 0.284 | < 0.001 |
| PT | 0.222 | < 0.001 |
| PTA | − 0.205 | < 0.001 |
| INR | 0.219 | < 0.001 |
| APTT | 0.125 | 0.001 |
| TT | − 0.217 | < 0.001 |
| FIB | 0.902 | < 0.001 |
| D-dimer | 0.416 | < 0.001 |
| FDP | 0.458 | < 0.001 |
| Triglycerides | − 0.160 | < 0.001 |
| HDL | − 0.089 | 0.025 |
| apoA | − 0.232 | < 0.001 |
| Lp (a) | 0.181 | < 0.001 |
SBP systolic blood pressure, DBP diastolic blood pressure, hs-CRP high-sensitivity C-reactive protein, NT-proBNP N-terminal pro-B type natriuretic peptide, FAR fibrinogen-to-albumin ratio, MLR monocyte-to-lymphocyte ratio, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, AST aspartate aminotransferase, eGFR estimated glomerular filtration rate, Na+ serum sodium, Ca2+ serum calcium, PT prothrombin time, PTA prothrombin time activity, INR international normalized ratio, APTT activated partial thromboplastin time, TT thrombin time, FIB fibrinogen, FDP fibrinogen degradation products, apoA apolipoprotein A, Lp(a) Lipoprotein(a)
The predictive implication of FAR
Univariate and multivariate logistic regression analyses and predictors of LVSD in ACS patients are presented in Additional file 1: Table S1. Univariate analyses showed that FAR, gender, HR, SBP, smoking history, hypertension, NT-proBNP, white blood cells, NLR, MLR, PLR, hs-CRP, ALT, AST, albumin, creatinine, cystatin C, eGFR, serum Na+, serum Ca2+, HCY, PT, PTA, INR, APTT, FIB, d-dimer, FDP, triglycerides, Lp(a), Gensini score, initial diagnosis (STEMI), Killip class(≥ III) and plaque property were risk factors for LVSD in ACS patients after PCI (all P < 0.05). FIB and albumin were not included in the multivariate analysis because FAR was calculated from them. Multivariate logistic regression showed that FAR, NT-proBNP, NLR, HCY and initial diagnosis (STEMI) were independent predictors of LVSD in ACS patients after adjustment for sex and other potential confounding factors (all P < 0.05) .
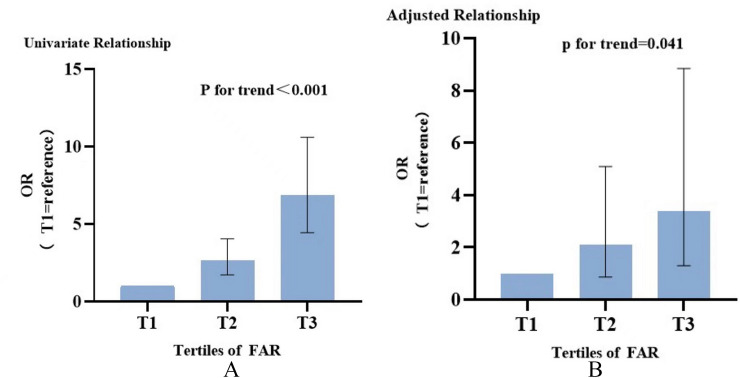
In univariate analysis, FAR as a continuous variable was associated with an OR of 1.037 (95% CI 1.029–1.046; P < 0.001). Four models, including variables of statistical significance (P < 0.05) and/or clinical importance, were constructed to assess the predictive potential of FAR for LVSD in multivariate logistic regression analysis. Adjustment for multiple confounding variables did not attenuate the correlation and FAR remained to be an independent risk predictor for endpoint (OR 1.030, 95% CI 1.011–1.049; P = 0.002) (Table 4). The incidence of the LVSD increased monotonically across the tertiles of FAR in crude model (P for trend ≤ 0.001) (Fig. 3A). Taking T1 as the reference, multivariate analysis revealed that T3 increased the ORs for the incidence of LVSD, while T2 did not reach the statistical significance (T2: OR 2.105, 95% CI 0.869–5.094; T3: OR 3.395, 95% CI 1.303–8.848) (Table 4).
Table 4.
Predictive value of FAR for LVEF in different logistic regression analysis
| FAR as a continuous variablea | |||
|---|---|---|---|
| OR | 95% CI | P value | |
| Crude model | 1.037 | 1.029–1.046 | < 0.001 |
| Model1 | 1.019 | 1.007–1.030 | 0.001 |
| Model2 | 1.026 | 1.011–1.042 | 0.001 |
| Model3 | 1.026 | 1.008–1.045 | 0.005 |
| Model4 | 1.030 | 1.011–1.049 | 0.002 |
| FAR as a categorical variableb | |||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Crude model | Reference | 2.628 (1.705–4.052) | < 0.001 | 6.854 (4.434–10.594) | < 0.001 |
| Model1 | Reference | 2.090 (1.223–3.571) | 0.007 | 2.140 (1.166–3.927) | 0.014 |
| Model2 | Reference | 2.431 (1.175–5.029) | 0.017 | 3.699 (1.649–8.298) | 0.002 |
| Model3 | Reference | 2.530 (1.094–5.854) | 0.030 | 3.738 (1.512–9.242) | 0.004 |
| Model4 | Reference | 2.105 (0.869–5.094) | 0.099 | 3.395 (1.303–8.848) | 0.012 |
Model 1: adjusted for age, sex (female), BMI, HR, SBP, DBP, smoking, hypertension, NT-proBNP, cardiac troponin T
Model 2: adjusted for variables included in Model 1 and white blood cells, NLR, MLR, PLR, hs-CRP, ALT, AST
Model 3: adjusted for variables included in Model 2 and cystatin C, Na+, Ca2+, homocysteine, PT, PTA, INR, APTT, d-dimer, FDP, triglycerides, apoA, Lp(a)
Model 4: adjusted for variables included in Model 3 and Gensini score, initial diagnosis (STEMI), Killip class (≥ III)
OR odds ratio, CI confidence interval
aThe OR was examined by per 1-unit increase of FAR
bThe OR was examined regarding T1 (the lowest) as reference
Fig. 3.
OR of impaired left ventricular ejection fraction according to FAR groups. A Crude model. B Model4 adjusted. Taking T1 as reference. OR Odds ratio, FAR fibrinogen-to-albumin ratio
The predictive effect of FAR for LVSD was greater than that of MLR, NLR, and PLR
The ROC curves predicting LVSD in ACS patients after PCI are illustrated in Fig. 4. FAR had the highest area under receiver operating characteristic curve (AUC) for prediction of LVSD compared with white blood cells, NLR, MLR and PLR (0.735, 0.680, 0.706, 0.688 and 0.594, respectively) (Table 5). The optimal value of FAR as an indicator for predicting the occurrence of LVSD was 79.16, which yielded a sensitivity of 59.6% and a specificity of 75.9%. The AUCs of FAR, FIB and albumin for the occurrence of LVSD are shown in Additional file 2: Table S2. The AUCs of FAR for predicting the occurrence of LVSD after adjusting for sex and hypertension are shown in Additional file 3: Table S3.
Fig. 4.
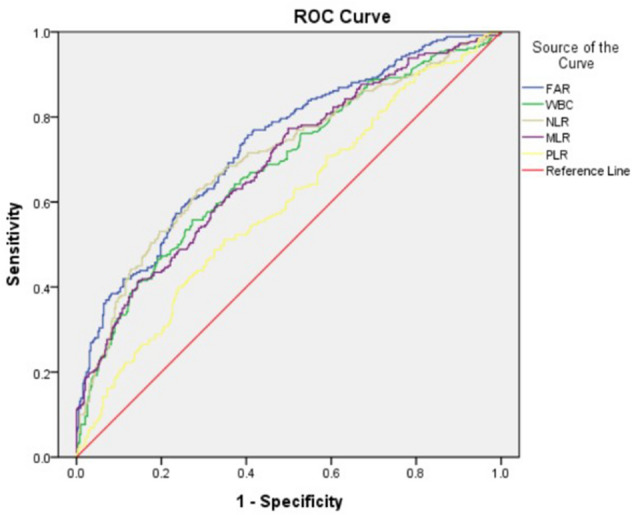
The receiver operating characteristic (ROC) curves based on FAR, white blood cells, NLR, MLR and PLR to predict LVSD in ACS patients after PCI. FAR fibrinogen-to-albumin ratio, WBC white blood cells, MLR monocyte-to-lymphocyte ratio, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio
Table 5.
AUCs of the inflammatory marker values for predicting the occurrence of LVSD
| Variables | AUC | 95%CI | P value | Cut-off | Specificity | Sensitivity |
|---|---|---|---|---|---|---|
| FAR | 0.735 | 0.696–0.774 | < 0.001 | 79.16 | 0.759 | 0.596 |
| White blood cells | 0.680 | 0.638–0.723 | < 0.001 | 8.09 | 0.556 | 0.728 |
| NLR | 0.706 | 0.664–0.748 | < 0.001 | 3.76 | 0.632 | 0.712 |
| MLR | 0.688 | 0.647–0.730 | < 0.001 | 0.21 | 0.773 | 0.491 |
| PLR | 0.594 | 0.549–0.638 | < 0.001 | 152.47 | 0.490 | 0.674 |
AUC area under receiver operating characteristic curve, LVSD left ventricular systolic dysfunction, CI confidence interval, FAR fibrinogen-to-albumin ratio, MLR monocyte-to-lymphocyte ratio, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio
Subgroup analysis
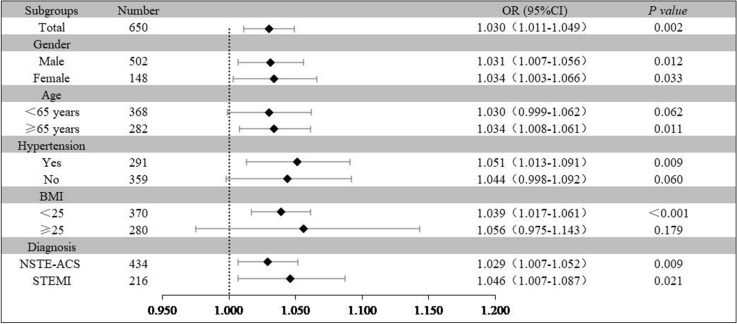
Relevant clinical variables like sex, age, BMI and clinical diagnosis were subject to post hoc subgroup analyses. The model adjusted in the subgroup analyses comprised all covariates used in Model 4 except for the variables used for stratification. Further evaluation of the predictive value of FAR for LVSD was performed in different subclasses. Increased FAR (per 1 unit) was consistently related to LVSD in various subgroups, including female or male, age ≥ 65 years, BMI < 25 kg/m2, with hypertension, NSTE-ACS or STEMI (Fig. 5). However, the results were not similar in patients aged below 65 years and patients without hypertension.
Fig. 5.
Logistic regression analysis evaluating predictive implication of FAR in various stratifications. OR was evaluated by 1-unit increase of FAR. OR Odds ratio, CI confidence interval, BMI body mass index, NSTE-ACS non-ST-segment elevation acute coronary syndromes, STEMI ST-segment elevation myocardial infarction
The nomogram model
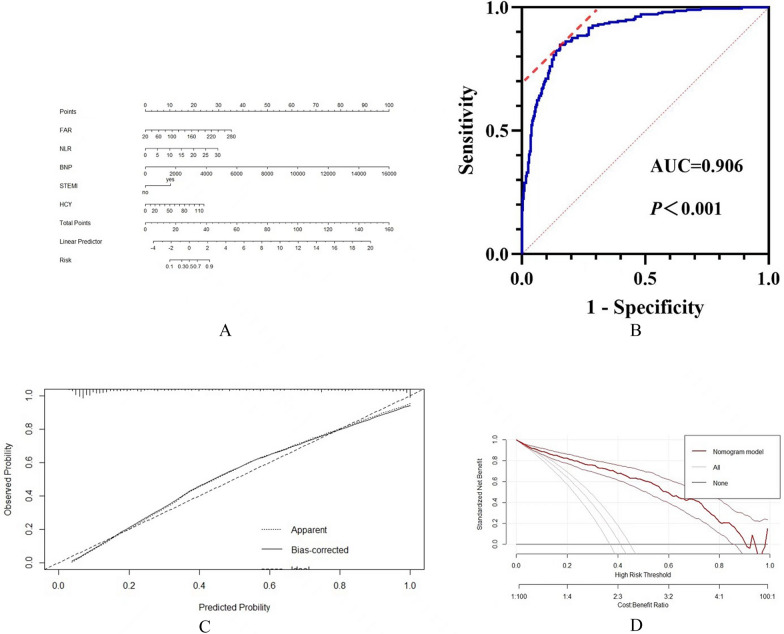
A nomogram was constructed to predict LVSD based on the final regression analysis (Fig. 6A). Furthermore, the AUC of the nomogram for LVSD were 0.906 (95%CI 0.881–0.932) in patients with ACS, indicting strong discrimination (Fig. 6B). A calibration curve of the nomogram is presented in Fig. 6C. The DCA indicated that the model showed better clinical benefit (Fig. 6D).
Fig. 6.
Construction and evaluation of nomogram. A Nomogram for prediction of LVSD. B C-index curves of the model. C Calibration curves of the model. D DCA curves of the model
Discussion
In the present study, the relationship between FAR and left ventricular systolic function was investigated in patients with ACS who underwent PCI with stent implantation. Patients with left ventricular systolic dysfunction had significantly higher FAR values than patients with preserved LVEF in the study population. FAR was a strong indicator of left ventricular dysfunction even after adjustment for confounders. In addition, the ROC curve demonstrated the predictive power of FAR was greater than that of NLR, followed by MLR, white blood cells and PLR for LVSD in ACS patients. To our knowledge, this is the first study exploring the predictive role of FAR to the LV function between ACS patients after PCI. These findings supported that inflammation indicators were effective markers for predicting LVSD in ACS patients. In addition, the results of this study may contribute to better risk stratification and management of patients with ACS.
Fibrinogen (FIB), an acute-phase protein, is synthesized primarily in hepatocytes and plays a crucial role in the physiology and pathophysiology of coagulation and inflammation [14]. Fibrinogen biosynthesis increases rapidly during the acute phase of inflammation, such as bacterial infection, severe trauma and surgery [15]. Elevated plasma fibrinogen levels are also involved in chronic, low-grade inflammatory processes, activation of platelets, adhesion molecule expression upregulation, stimulation of angiogenesis and macrophages infiltration enhancement, which consequently aggravate atherosclerotic plaque progression [16]. Increased plasma fibrinogen concentration been confirmed the cause of the development of atherosclerotic lesions. Numerous observational studies identified that increased plasma fibrinogen concentrations were closely associated with CVD. Yuan et al. reported that plasma FIB was independently associated with long-term risk of all-cause and cardiac mortality in CAD patients after PCI [17]. Jiang et al. indicated fibrinogen concentration was associated with 2-year all-cause mortality in patients undergoing PCI [18]. Many cardiovascular risk factors can reversely lead to increased plasma concentration of fibrinogen, like age, diabetes, hypertension, obesity, lipid disorders, metabolic syndrome, smoking and alcohol consumption [19]. Albumin is synthesized in the liver, and the synthesis ability is affected by both nutrition and inflammation condition [20]. Malnutrition and inflammation are considered to play a major role in occurrence of hypoalbuminemia. Serum albumin has many physiological properties, such as anti-inflammatory activity, antioxidant, anticoagulant, antiplatelet aggregation and maintenance of capillary membrane stability [21]. Evidence has emerged that hypoalbuminemia is a powerful prognostic marker in the general population and in patients with cardiovascular diseases. After adjustment for traditional cardiovascular risk factors, serum albumin levels remained inversely associated with ischemic heart disease, heart failure and stroke [22–24]. Also, hypoalbuminemia is a powerful predictor of the cardiovascular prognosis in patients with CVD. A previous study had shown that lower serum albumin levels were associated with adverse cardiac events in patients with CAD after PCI [25]. Given that both plasma FIB and albumin showed strong correlation with adverse cardiovascular events, subsequent studies are warranted to evaluate whether FAR could be helpful in identifying high-risk populations in ACS patients undergoing PCI.
Since fibrinogen and albumin are positively and negatively correlated with systemic inflammation, respectively, researchers have proposed the hypothesis that FAR may be more closely related to inflammation than fibrinogen or albumin alone. Previous studies have confirmed the combination of fibrinogen and albumin parameters represent a more reliable and efficient indicator for the prognosis of multiple tumors and cardiovascular events than individual parameter separately (A–E). FAR was shown to be an independent predictor of the presence and severity of CAD among angina patients [26]. Oğuz et al. demonstrated that FAR was significantly associated with SYNTAX score in STEMI patients after PCI [27]. Furthermore, Xiao et al. analyzed 475 patients with STEMI and determined that the FAR was an independent prognostic factor for all-cause mortality in the population [28]. Recent research also reported that the FAR was an independent predictor of long-term outcomes in patients with NSTE-ACS who underwent PCI [29]. Consistent with the results of the above studies, FAR has been shown to be more powerful than fibrinogen or albumin alone in predicting the prognosis of patients with malignant tumors. Qiang et al. indicated that FAR was a novel prognostic indicator for patients with stage IB-IIA cervical cancer [30]. High FAR had been shown to be inversely associated with overall survival for locally advanced or metastatic pancreatic cancer [31]. In addition, FAR was reported to be a valuable marker for predicting long-term adverse prognosis in patients with gastric cancer treated with first-line chemotherapy, and its prognostic value was superior to that of fibrinogen or albumin alone [32].
Recent studies revealed that elevated NLR was an independent predictor for LVSD in ACS patients [33, 34]. NLR was demonstrated negatively associated with LVEF in patients with NSTE-ACS [33]. Orhan et.al found that NLR was a sensitive and specific predictor of impaired LV systolic dysfunction [34]. Adem et al. reported high PLR was a strong and independent predictor for LVSD in NSTE-ACS patients [35]. It is previously shown that elevated WBC levels are an independent predictor for the occurrence of LVSD after ACS regardless of several confounding factors [36]. Consistent with these results, we also found that NLR was an independent predictor of LVSD after adjusting for multiple covariates in ACS patients undergoing PCI.
Left ventricular dysfunction has been proved as the arguably powerful predictor of morbidity and mortality in ACS patients [4]. There are multiple mechanisms contributing to adverse left ventricular remodeling after acute myocardial infarction, such as large infarct size, excessive inflammatory response, irreversible microvascular disturbance, extracellular matrix changes, collagen deposition, fibroblast aggregation, eccentric hypertrophy, oxidative stress and neurohormonal activation [37, 38]. Our findings implied that elevated FAR may be partly involved in potential mechanism of left ventricular remodeling after ACS, resulting in decreased LVEF. Previous studies showed that higher FAR levels were significantly and independently related to the presence of angiographic coronary slow flow and no-reflow [39, 40]. The occurrence of coronary no-reflow may be associated with diffuse atherosclerosis, increased systemic inflammatory load, platelet dysfunction and impaired endothelial function, leading to coronary microvascular dysfunction. Therefore, we proposed that higher FAR may have caused worse microvascular perfusion, thereby affecting left ventricular functions. Considering these findings, it is reasonable to further investigate the underlying mechanisms for FAR in left ventricular remodeling.
The present study has some limitations. Firstly, the retrospective study was based on a single-center trial with a limited sample size and may not be generalized to other cohorts. Secondly, residual confounding by other unmeasured covariates cannot be excluded despite the attempt to perform potential risk factors adjustment. Finally, the measurement of echocardiogram was performed only once within 24 h after admission and may have failed to measure changes in LVEF after revascularization. Further multi-centric studies with larger populations are needed to clarify potential association between FAR in patients with left ventricular systolic dysfunctions.
Conclusions
FAR is an affordable and reliable predictor of LV systolic dysfunction in ACS patients undergoing PCI and the predictive power of FAR is greater than that of MLR, NLR, and PLR. Thus, the practice of using FAR on admission may help identify high-risk patients and relevant treatments.
Supplementary Information
Additional file 1: Table S1. Univariate and multivariate analysis and predictors of LVSD in ACS patients.
Additional file 2: Table S2. AUCs of FAR, FIB and albumin predicting the occurrence of LVSD.
Additional file 3: Table S3. AUCs of FAR predicting the occurrence of LVSD after adjusting for sex and hypertension.
Acknowledgements
Not applicable.
Abbreviations
- CAD
Coronary artery disease
- ACS
Acute coronary syndrome
- LVSD
Left ventricular systolic dysfunction
- LVEF
Left ventricular ejection fraction
- NLR
Neutrophil-to-lymphocyte ratio
- PLR
Platelet-to-lymphocyte ratio
- MLR
Monocyte-to-lymphocyte ratio
- PCI
Percutaneous coronary intervention
- UA
Unstable angina
- NSTEMI
Non-ST-segment elevation myocardial infarction
- STEMI
ST-segment elevation myocardial infarction
- FIB
Fibrinogen
- CVD
Cardiovascular disease
Author contributions
XW, YH, and GT designed the study. XW, YH, HL, KK and TZ analyzed and interpreted the data. XW, YH and HL drafted the manuscript. XW, YH, HL, CL, and GT revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Nature Science Foundation of China (Grant No. 81873513), Key Projects of Shaanxi Science and Development Plan (Grant No. 2018ZDXM-SF-049).
Availability of data and materials
All data used and analyzed in this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethical Committee of the First Affiliated Hospital of Xi’an Jiaotong University and conducted in line with the principles of the Declaration of Helsinki. The Institutional Review Board at The First Affiliated Hospital of Xi’an JiaoTong University approved the use of the deidentified data for this study and waived consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuan Wang, Yi Hu and Hao Luan contributed equally to this work and share first authorship
References
- 1.Eisen A, Giugliano RP, Braunwald E. Updates on acute coronary syndrome: a review. JAMA Cardiol. 2016;1(6):718–730. doi: 10.1001/jamacardio.2016.2049. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JG, Torabi A, Khan NK. Epidemiology and management of heart failure and left ventricular systolic dysfunction in the aftermath of a myocardial infarction. Heart. 2005;91:ii7. doi: 10.1136/hrt.2005.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamblin N, Meurice T, Tricot O, Lemesle G, Deneve M, de Groote P, et al. Effect of left ventricular systolic dysfunction on secondary medical prevention and clinical outcome in stable coronary artery disease patients. Arch Cardiovasc Dis. 2017;110(1):35–41. doi: 10.1016/j.acvd.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Bosch X, Théroux P. Left ventricular ejection fraction to predict early mortality in patients with non-ST-segment elevation acute coronary syndromes. Am Heart J. 2005;150(2):215–220. doi: 10.1016/j.ahj.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Yahud E, Tzuman O, Fink N, Goldenberg I, Goldkorn R, Peled Y, et al. Trends in long-term prognosis according to left ventricular ejection fraction after acute coronary syndrome. J Cardiol. 2020;76(3):303–308. doi: 10.1016/j.jjcc.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Balta S, Kurtoglu E, Kucuk U, Demirkol S, Ozturk C. Neutrophil-lymphocyte ratio as an important assessment tool. Expert Rev Cardiovasc Ther. 2014;12(5):537–538. doi: 10.1586/14779072.2014.902309. [DOI] [PubMed] [Google Scholar]
- 7.Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97(7):993–996. doi: 10.1016/j.amjcard.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Sun XP, Li J, Zhu WW, Li DB, Chen H, Li HW, et al. Impact of platelet-to-lymphocyte ratio on clinical outcomes in patients with ST-segment elevation myocardial infarction. Angiology. 2017;68(4):346–353. doi: 10.1177/0003319716657258. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Ma X, Shao Q, Yang Z, Wang Y, Gao F, et al. Prognostic impact of multiple lymphocyte-based inflammatory indices in acute coronary syndrome patients. Front Cardiovasc Med. 2022;3(9):811790. doi: 10.3389/fcvm.2022.811790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Xu C, Liu J, Bai X, Li R, Wang L, et al. Baseline plasma fibrinogen is associated with haemoglobin A1c and 2-year major adverse cardiovascular events following percutaneous coronary intervention in patients with acute coronary syndrome: a single-centre, prospective cohort study. Cardiovasc Diabetol. 2019;18(1):52. doi: 10.1186/s12933-019-0858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González-Pacheco H, Amezcua-Guerra LM, Sandoval J, Martínez-Sánchez C, Ortiz-León XA, Peña-Cabral MA, et al. Prognostic implications of serum albumin levels in patients with acute coronary syndromes. Am J Cardiol. 2017;119(7):951–958. doi: 10.1016/j.amjcard.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 12.Liu LS. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39(7):579–615. [PubMed] [Google Scholar]
- 13.Section of Interventional Cardiology of Chinese Society of Cardiology of Chinese Medical Association, Specialty Committee on Prevention and Treatment of Thrombosis of Chinese College of Cardiovascular Physicians, Editorial Board of Chinese Journal of Cardiology. Chinese guideline for percutaneous coronary intervention. Chin J Cardiol. 2016;44(5):382–400. [DOI] [PubMed]
- 14.Vilar R, Fish RJ, Casini A, Neerman-Arbez M. Fibrin(ogen) in human disease: both friend and foe. Haematologica. 2020;105(2):284–296. doi: 10.3324/haematol.2019.236901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen T, Kierulf P, Sandset PM, Klingenberg O, Joø GB, Godal HC, et al. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb Haemost. 2007;97(5):822–829. doi: 10.1160/TH07-01-0039. [DOI] [PubMed] [Google Scholar]
- 16.Kryczka KE, Kruk M, Demkow M, Lubiszewska B. Fibrinogen and a triad of thrombosis, inflammation, and the renin-angiotensin system in premature coronary artery disease in women: a new insight into sex-related differences in the pathogenesis of the disease. Biomolecules. 2021;11(7):1036. doi: 10.3390/biom11071036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan D, Jiang P, Zhu P, Jia S, Zhang C, Liu Y, et al. Prognostic value of fibrinogen in patients with coronary artery disease and prediabetes or diabetes following percutaneous coronary intervention: 5-year findings from a large cohort study. Cardiovasc Diabetol. 2021;20(1):143. doi: 10.1186/s12933-021-01335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang P, Gao Z, Zhao W, Song Y, Tang XF, Xu JJ, et al. Relationship between fibrinogen levels and cardiovascular events in patients receiving percutaneous coronary intervention: a large single-center study. Chin Med J (Engl) 2019;132(8):914–921. doi: 10.1097/CM9.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaptoge S, White IR, Thompson SG, Wood AM, Lewington S, Lowe GD, et al. Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: individual participant meta-analysis of 154,211 adults in 31 prospective studies: the fibrinogen studies collaboration. Am J Epidemiol. 2007;166(8):867–879. doi: 10.1093/aje/kwm191. [DOI] [PubMed] [Google Scholar]
- 20.Kirsch R, Frith L, Black E, Hoffenberg R. Regulation of albumin synthesis and catabolism by alteration of dietary protein. Nature. 1968;217(5128):578–579. doi: 10.1038/217578a0. [DOI] [PubMed] [Google Scholar]
- 21.Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12. doi: 10.1016/j.ejim.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Nelson JJ, Liao D, Sharrett AR, Folsom AR, Chambless LE, Shahar E, et al. Serum albumin level as a predictor of incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151(5):468–477. doi: 10.1093/oxfordjournals.aje.a010232. [DOI] [PubMed] [Google Scholar]
- 23.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Tang WW, Methvin A, Smith AL, et al. Serum albumin concentration and heart failure risk The Health, Aging, and Body Composition Study. Am Heart J. 2010;160(2):279–285. doi: 10.1016/j.ahj.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu WH, Dong C, Rundek T, Elkind MS, Sacco RL. Serum albumin levels are associated with cardioembolic and cryptogenic ischemic strokes: Northern Manhattan Study. Stroke. 2014;45(4):973–978. doi: 10.1161/STROKEAHA.113.003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada H, Dohi T, Miyauchi K, Shitara J, Endo H, Doi S, et al. Impact of serum albumin levels on long-term outcomes in patients undergoing percutaneous coronary intervention. Heart Vessels. 2017;32(9):1085–1092. doi: 10.1007/s00380-017-0981-8. [DOI] [PubMed] [Google Scholar]
- 26.Deveci B, Gazi E. Relation between globulin, fibrinogen, and albumin with the presence and severity of coronary artery disease. Angiology. 2021;72(2):174–180. doi: 10.1177/0003319720959985. [DOI] [PubMed] [Google Scholar]
- 27.Karahan O, Acet H, Ertaş F, Tezcan O, Çalişkan A, Demir M, et al. The relationship between fibrinogen to albumin ratio and severity of coronary artery disease in patients with STEMI. Am J Emerg Med. 2016 Jun;34(6):1037–42. [DOI] [PubMed]
- 28.Xiao L, Jia Y, Wang X, Huang H. The impact of preoperative fibrinogen-albumin ratio on mortality in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Clin Chim Acta. 2019;493:8–13. doi: 10.1016/j.cca.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Tang C, Luo E, Qin Y, Wang D, Yan G. Relation of fibrinogen-to-albumin ratio to severity of coronary artery disease and long-term prognosis in patients with non-ST elevation acute coronary syndrome. Biomed Res Int. 2020;17(2020):1860268. doi: 10.1155/2020/1860268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An Q, Liu W, Yang Y, Yang B. Preoperative fibrinogen-to-albumin ratio, a potential prognostic factor for patients with stage IB-IIA cervical cancer. BMC Cancer. 2020;20(1):691. doi: 10.1186/s12885-020-07191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang L, Yan FH, Liu C, Chen J, Wang D, Zhang CH, et al. Systemic inflammatory biomarkers, especially fibrinogen to albumin ratio, predict prognosis in patients with pancreatic cancer. Cancer Res Treat. 2021;53(1):131–139. doi: 10.4143/crt.2020.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Wang Z, Xiao J, Zhang Z, Li H, Wang Y, et al. Prognostic value of fibrinogen-to-albumin ratio in patients with gastric cancer receiving first-line chemotherapy. Oncol Lett. 2020;20(4):10. doi: 10.3892/ol.2020.11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bekler A, Erbag G, Sen H, Gazi E, Ozcan S. Predictive value of elevated neutrophil-lymphocyte ratio for left ventricular systolic dysfunction in patients with non-ST-elevated acute coronary syndrome. Pak J Med Sci. 2015;31(1):159–163. doi: 10.12669/pjms.311.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doğdu O, Akpek M, Yarlıoğlueş M, Kalay N, Ardıç I, Elçik D, et al. Relationship between hematologic parameters and left ventricular systolic dysfunction in stable patients with multi-vessel coronary artery disease. Turk Kardiyol Dern Ars. 2012;40:706–713. doi: 10.5543/tkda.2012.82429. [DOI] [PubMed] [Google Scholar]
- 35.Bekler A, Gazi E, Yılmaz M, Temiz A, Altun B, Barutçu A, et al. Could elevated platelet-lymphocyte ratio predict left ventricular systolic dysfunction in patients with non-ST elevated acute coronary syndrome? Anatol J Cardiol. 2015;15(5):385–390. doi: 10.5152/akd.2014.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aggelopoulos P, Chrysohoou C, Pitsavos C, Papadimitriou L, Liontou C, Panagiotakos D, et al. Comparative value of simple inflammatory markers in the prediction of left ventricular systolic dysfunction in postacute coronary syndrome patients. Mediators Inflamm. 2009;2009:826297. doi: 10.1155/2009/826297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt AS, Ambrosy AP, Velazquez EJ. Adverse remodeling and reverse remodeling after myocardial infarction. Curr Cardiol Rep. 2017;19(8):71. doi: 10.1007/s11886-017-0876-4. [DOI] [PubMed] [Google Scholar]
- 38.Westman PC, Lipinski MJ, Luger D, Waksman R, Bonow RO, Wu E, et al. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2016;67(17):2050–2060. doi: 10.1016/j.jacc.2016.01.073. [DOI] [PubMed] [Google Scholar]
- 39.Kayapinar O, Ozde C, Kaya A. Relationship between the reciprocal change in inflammation-related biomarkers (fibrinogen-to-albumin and hsCRP-to-albumin ratios) and the presence and severity of coronary slow flow. Clin Appl Thromb Hemost. 2019;25:1076029619835383. doi: 10.1177/1076029619835383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Yang J, Ji Y, Wang S, Wang T, Wang F, et al. Usefulness of fibrinogen-to-albumin ratio to predict no-reflow and short-term prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessels. 2019;34(10):1600–1607. doi: 10.1007/s00380-019-01399-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Univariate and multivariate analysis and predictors of LVSD in ACS patients.
Additional file 2: Table S2. AUCs of FAR, FIB and albumin predicting the occurrence of LVSD.
Additional file 3: Table S3. AUCs of FAR predicting the occurrence of LVSD after adjusting for sex and hypertension.
Data Availability Statement
All data used and analyzed in this study are available from the corresponding author on reasonable request.