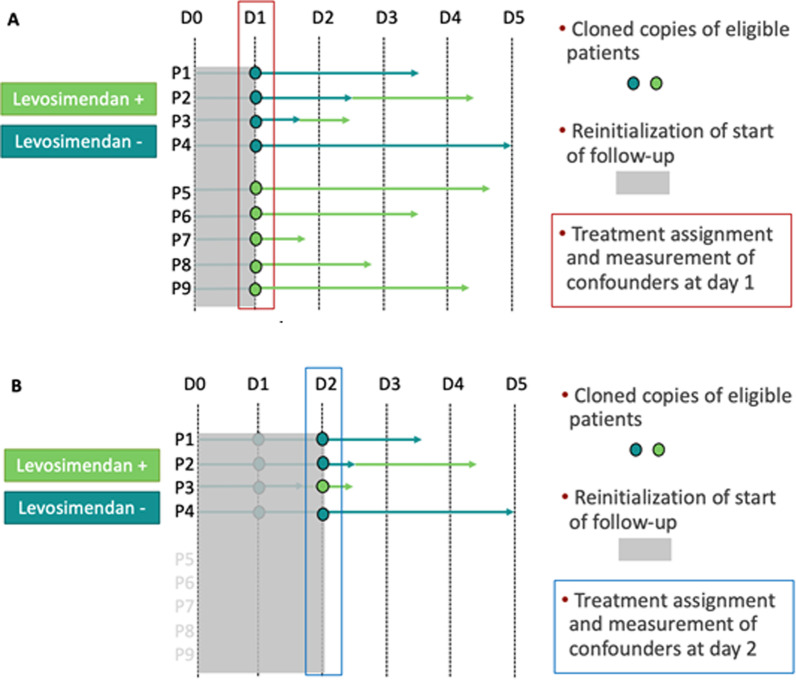
Fig. 1.
Sequential emulation of the target trial. The target trial (as defined in Additional file 1: Table S2) was emulated several times in a sequence of nested trials, to make coincide treatment assignment and start of follow-up, thus avoiding immortal-time bias. This figure describes the construction of the observations in each nested trial with their respective treatment assignment, measurement of confounders, and follow-up. A hypothetical cohort is presented for simplicity. Panel A: The figure depicts the construction of the first emulated trial. At day 0 (first day of ECMO support), no patient received levosimendan; thus, the target trial was not emulated as the comparison was not feasible between patient receiving and not receiving levosimendan. At day 1 (D1), five patients (P5 to P9) started levosimendan, and so could be compared to the four patients (P1 to P4) that did not receive levosimendan. Their treatment assignment is in line with the received strategy at the day of the target trial emulation (here day 1). In addition, baseline confounders for the first nested trial are measured on day 1 (red rectangle), that is, day 1 since beginning of ECMO. Follow-up is restarted at day 1 for the first emulated trial to make coincide eligibility, treatment assignment and time-zero. Panel B: The figure depicts the construction of the second emulated trial. At day 2 after beginning of ECMO support, three patients did not receive levosimendan (P1, P2, and P4) and one patient received levosimendan (P3). Patients that received levosimendan in the previous nested trial at day 1 are not eligible anymore, as defined in the target trial protocol (new-user design). As for day 1 nested trial, treatment assignment of cloned copies of eligible patients is based on the treatment received the day of the emulated trial (blue rectangle) and baseline confounders for the second nested trial are measured on day 2. Follow-up is again restarted on the day 2 of the nested trial. Each of these nested emulated trials data is then stacked in a unique dataset, built with cloned copies of participants with assigned treatment, reset follow-up and baseline confounders in each nested trial. A multivariable Cox model is then fitted on the stacked cohort of observations and adjusted for confounders measured at ICU admission and at baseline of each nested trial (lactatemia, ECMO output, VIS, and SOFA score)