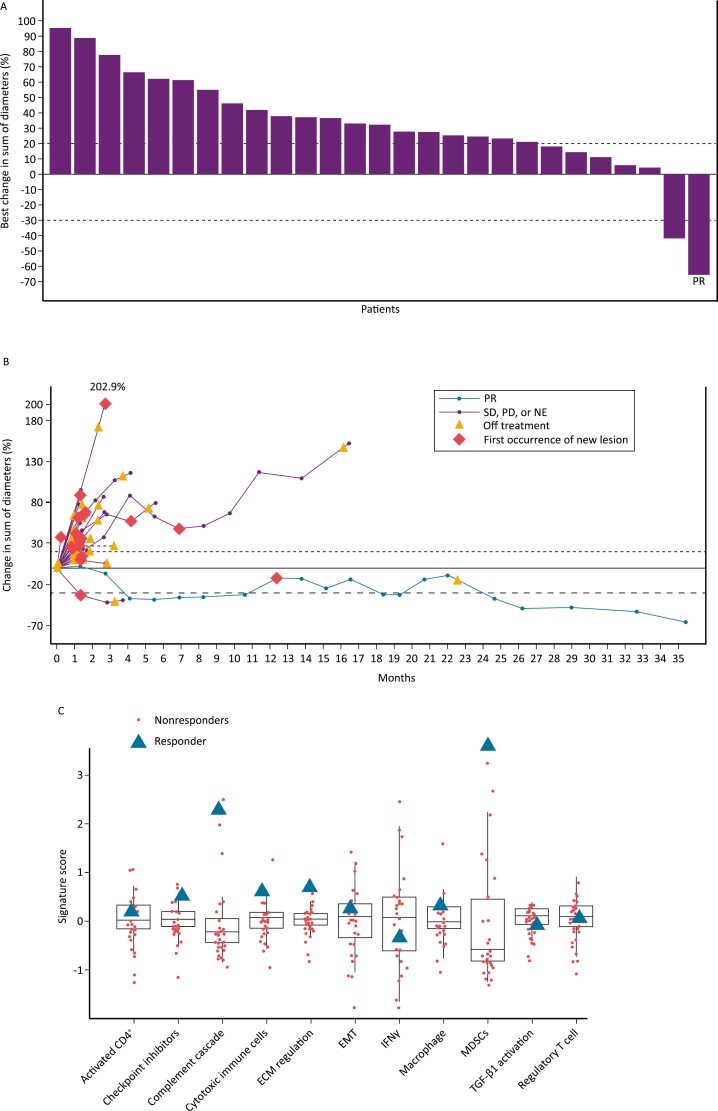
Figure 1.
(A) Percent change in diameter from baseline in target lesions, as assessed by IRC per RECIST 1.1. (B) Percent change in target lesion diameter from baseline to best postbaseline value, as assessed by IRC per RECIST 1.1. (C) Patient gene signatures by RNAseq. The patient with a PR had colorectal cancer that was microsatellite stable, mesenchymal subtype (consensus molecular subtype 4), KRAS mutant, and PD-L1 positive (PD-L1 ≥ 1% tumor cell expression by immunohistochemistry). A higher signature score for complement cascade and myeloid-derived suppressor cells was observed. Of 32 patients who received bintrafusp alfa, 2 patients were not evaluable by IRC due to no IRC review (n = 1) and SD (or better) of insufficient duration (<6 weeks after start date without further evaluable tumor assessment) (n = 1). Abbreviations: IRC, independent review committee; RECIST, Response Evaluation Criteria in Solid Tumors; PR, partial response; SD, stable disease; PD; progressive disease; NE, not evaluable; ECM, extracellular matrix; EMT, epithelial to mesenchymal transition; IFNγ, interferon γ; MDSC, myeloid-derived suppressor cell; TGF-β, transforming growth factor β.