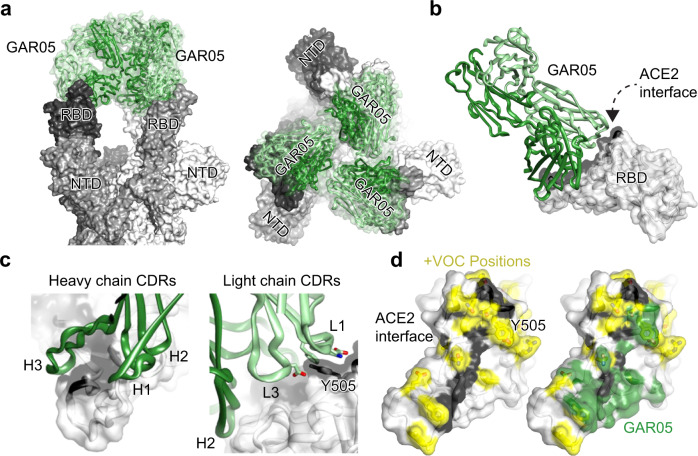
Fig. 2. Structural characterization of the broadly neutralizing class 1 antibody GAR05.
a Cryo-EM structure of GAR05 Fab bound to trimeric spike (3.27 Å resolution) showing full antibody Fab occupancy of all the RBDs in the “up” conformation. Two perspectives are shown b Structure of GAR05 bound to SARS-CoV-2 RBD based on the X-ray crystal structure, outlining the antibody bound to the ACE2 “saddle” of the RBD (ACE2 interface shaded black). c Interaction of the CDR regions of the VH and VL domains with the RBD saddle and the Y505 side chain of the RBD. d Comparison of the ACE2 interface on the surface of RBD and the GAR05 epitope, showing high overlap within the ACE2 saddle region. The large, buried surface area of GAR05 (1275 Å2) indeed blankets many key residues identified in VOCs, colored in yellow, yet remarkably still binds all VOCs with high affinity.