Fig. 4.
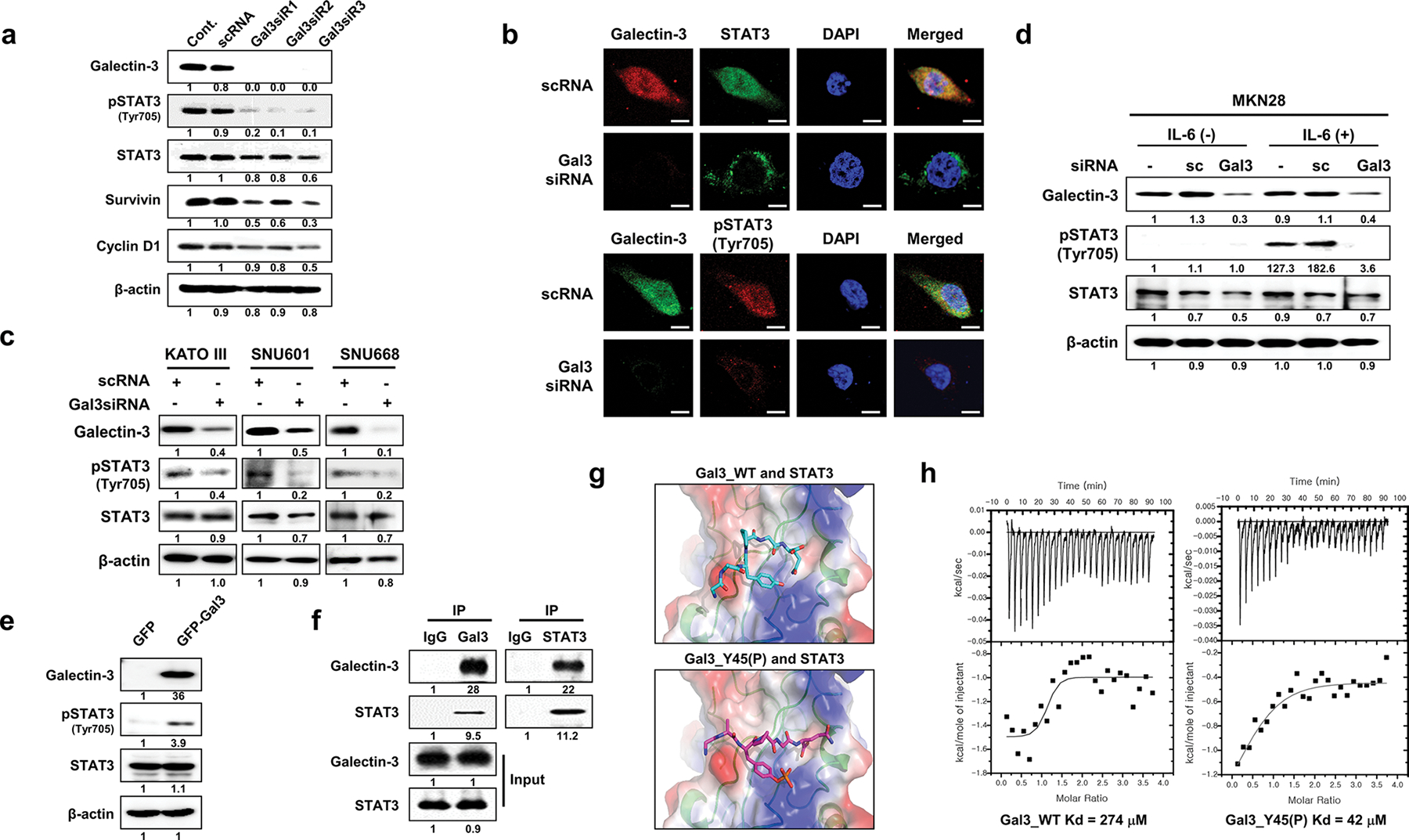
Galectin-3 depletion reduces Tyr705 phosphorylation of STAT3 and its nuclear localization through direct interaction with STAT3. a Levels of the indicated proteins in nuclear and cytosolic fractions of AGS cells after galectin-3 silencing with galectin-3 siRNA as measured by western blotting. b Immunocytochemical analysis of the subcellular localization of FITC-STAT3 and Cy5-pSTAT3(Tyr705) in FITC (up) or Cy5 (down)-galectin-3-silenced AGS cells. DAPI was used to visualize the nuclei (blue). Scale bars, 10 μm. c After transfection of three gastric cancer cell lines, KATOIII, SNU 601, and SNU668, with galectin-3 siRNA, the expression levels of galectin-3, phosphorylated STAT3 (Tyr705), and STAT3 were assessed by western blotting. β-Actin was used as a loading control. d After galectin-3-specific siRNA transfection, IL-6 cytokine was subjected for 6 h in MKN28 cells, and the expression levels of galectin-3, phosphorylated STAT3(Tyr705), and STAT3 were detected by western blotting. e Evaluation of galectin-3, STAT3, and pSTAT3(Tyr705) levels in SNU-638 cells transfected with pcDNA3.1/NT-GFP or pcDNA3.1/NT-GFP-gal3. f Immunoprecipitation (IP) with an anti-galectin-3 or STAT3 antibody to detect galectin-3 interaction with STAT3 in AGS cells. g Model structures of a STAT3/WT galectin-3 peptide (upper panel) with a STAT3/Y45(P) galectin-3 peptide (lower panel). h Binding affinities of STAT3 to a WT galectin-3 peptide (left) and Y45(P) galectin-3 peptide as measured by ITC analysis
