Figure 5. Structural ensembles of the RBD-CRD interactions with KD for B-Raf autoinhibition in the absence of the14-3-3 dimer.
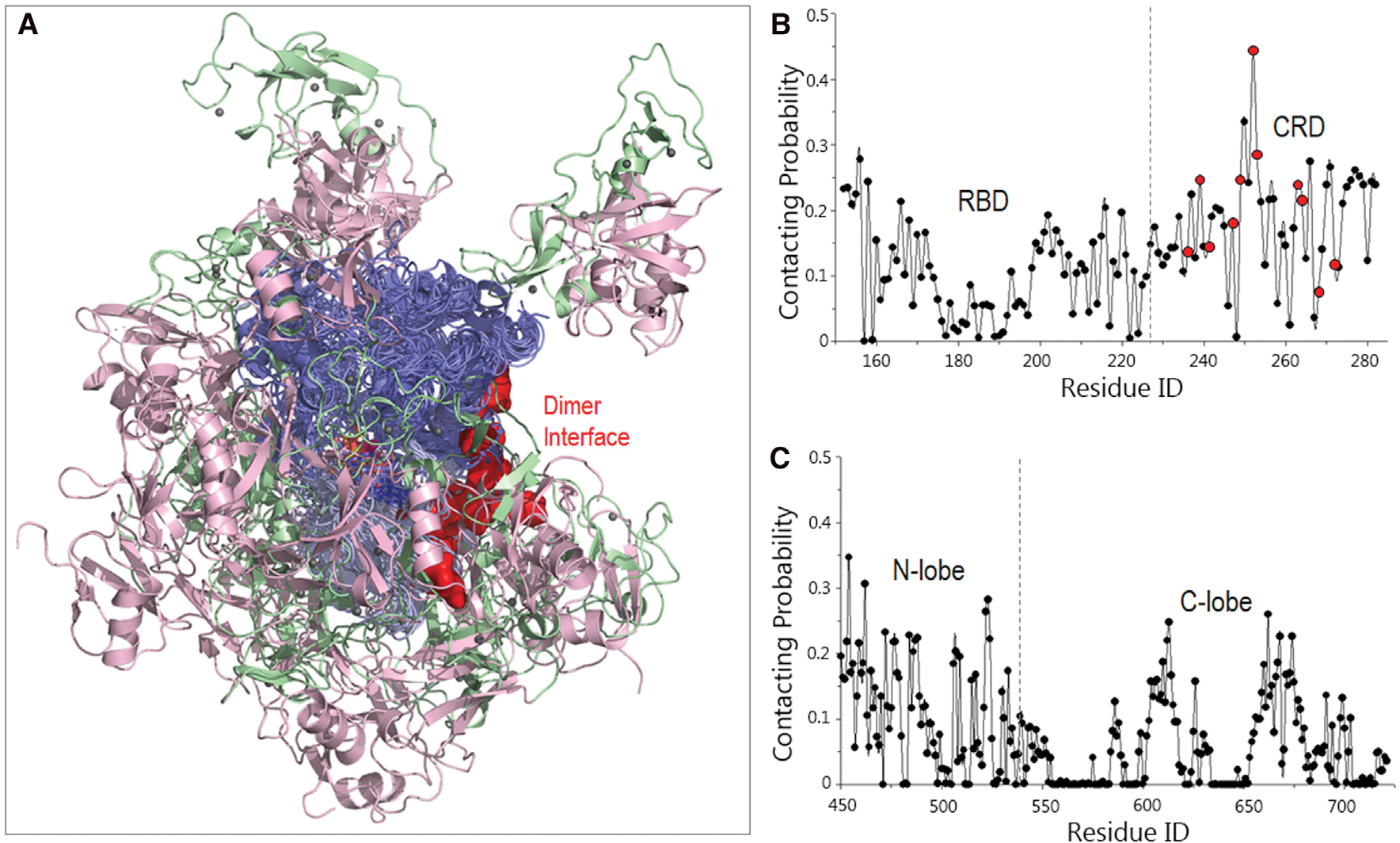
(A) The superimposed structures indicate that the RBD-CRD exhibits a potential to interact with the KD.
(B and C) The residue-based contacting probabilities of (B) RBD-CRD and (C) KD suggest a preference of CRD and KD’s N lobe for the RBD-CRD interactions with KD. The implicated activating residues in CRD for the RBD-CRD interactions with KD are highlighted by red points.
