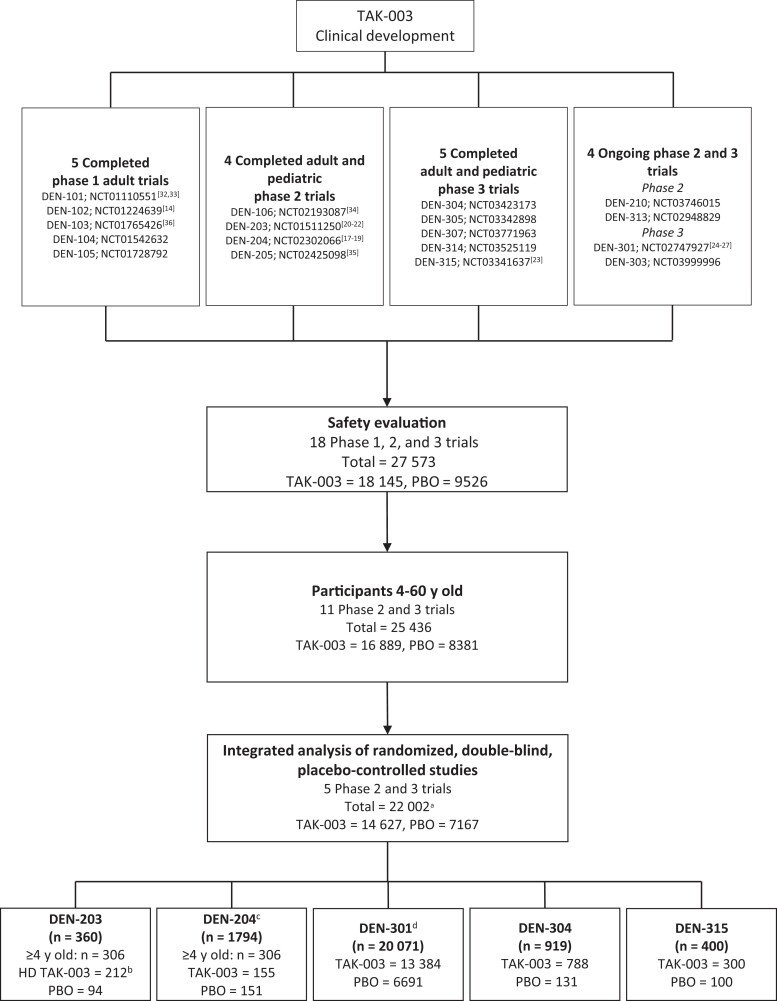
Figure 2.
Overview of TAK-003 clinical development program and trials included in the current integrated safety evaluation of placebo (PBO)–controlled randomized controlled trials. aAll participants received the commercially intended formulation of TAK-003 except 212 participants in DEN-203 who received a non–commercially-intended formulation of TAK-003 (HD TAK-003). bA high-dose formulation of TAK-003 (HD TAK-003), differing from the commercially intended formulation, was used in this trial: therefore, only PBO recipients ≥4 years old were included in the integrated analysis. cOnly group 1 (TAK-003 2-dose regimen given 3 months apart) and group 4 (PBO) participants ≥4 years old were included in the integrated analysis. Reactogenicity and unsolicited adverse events were recorded for a randomly selected subset of 562 of 1794 total participants in this trial. d4 participants (3 assigned to 2 doses of TAK-003 and 1 assigned to 2 doses of PBO) erroneously received 1 dose of PBO and 1 dose of TAK-003. Reactogenicity and unsolicited adverse events were recorded for a randomly selected subset of 3992 of 20 071 total participants in this trial.