Abstract
Background
Treatment success rates for multidrug-resistant tuberculosis (MDR-TB) remain low globally. Availability of newer drugs has given scope to develop regimens that can be patient-friendly, less toxic, with improved outcomes. We proposed to determine the effectiveness of an entirely oral, short-course regimen with bedaquiline and delamanid in treating MDR-TB with additional resistance to fluoroquinolones (MDR-TBFQ+) or second-line injectable (MDR-TBSLI+).
Methods
We prospectively determined the effectiveness and safety of combining 2 new drugs with 2 repurposed drugs—bedaquiline, delamanid, linezolid, and clofazimine—for 24–36 weeks in adults with pulmonary MDR-TBFQ+ and/or MDR-TBSLI+. The primary outcome was a favorable response at end of treatment, defined as 2 consecutive negative cultures taken 4 weeks apart. The unfavorable outcomes included bacteriologic or clinical failure during the treatment period.
Results
Of the 165 participants enrolled, 158 had MDR-TBFQ+. At the end of treatment, after excluding 12 patients due to baseline drug susceptibility and culture negatives, 139 of 153 patients (91%) had a favorable outcome. Fourteen patients (9%) had unfavorable outcomes: 4 deaths, 7 treatment changes, 2 bacteriological failures, and 1 withdrawal. During treatment, 85 patients (52%) developed myelosuppression, 69 (42%) reported peripheral neuropathy, and none had QTc(F) prolongation >500 ms. At 48 weeks of follow-up, 131 patients showed sustained treatment success with the resolution of adverse events in the majority.
Conclusions
After 24–36 weeks of treatment, this regimen resulted in a satisfactory favorable outcome in pulmonary MDR-TB patients with additional drug resistance. Cardiotoxicity was minimal, and myelosuppression, while common, was detected early and treated successfully.
Clinical Trials Registration
ClinicalTrials Registry of India (CTRI/2019/01/017310).
Keywords: drug-resistant tuberculosis, short-course, delamanid, linezolid, effectiveness
Highly drug-resistant pulmonary tuberculosis can be successfully treated with an entirely oral, short-course regimen of bedaquiline, delamanid, linezolid, and clofazimine. Toxicities can be identified early and corrected, thus reducing the duration, pill, and financial burden to the patient and program.
Drug resistance significantly threatens global tuberculosis (TB) care and control. Globally, in 2020, 71% (2.1/3.0 million) of patients with bacteriologically confirmed pulmonary TB were tested for rifampicin resistance, of whom 132 222 cases of multidrug-resistant/rifampicin-resistant TB (MDR/RR-TB) and 25 681 cases of pre-extensively or extensively drug-resistant (DR) TB were detected [1]. Treatment success rates for MDR/RR-TB remain at 59%, with a considerable gap between the estimated global incidence and people initiating treatment [1]. This situation will improve as newer drugs like bedaquiline (BDQ)– and delamanid (DLM)–based regimens become available and used widely.
Studies have shown that BDQ or DLM, when added to an optimized background regimen (OBR), showed an excellent response to treatment and were associated with a lower risk of death [2–5]. Few other antibacterial drugs with anti-mycobacterial properties, like linezolid (LZD) and clofazimine (CFZ), are being repurposed for the management of DR-TB [6]. Studies have demonstrated that LZD hastens culture conversion, treatment success, and regimen effectiveness when used for 6 months in patients with DR-TB [5, 7, 8]. Clofazimine, with its concentration-dependent anti-mycobacterial, pro-oxidative, and anti-inflammatory properties, when added to the treatment regimen, has shown reduced lung bacterial load and relapse rates in mice, raising the potential for shortening the treatment duration [9, 10].
To effectively manage DR-TB, country programs can consider combining newer drugs with repurposed drugs rather than using them individually with OBR [11]. Regimens with a BDQ and DLM combination have shown good culture conversion with no additive or synergistic QTc-prolongation after 6 months of treatment [12–14]. Also, the World Health Organization (WHO) has regrouped anti-TB drugs and recommended including drugs from both group A and B to have at least 4 effective medicines in a regimen [5]. In India, the cure rate of patients with MDR-TB with additional resistance to fluoroquinolones (MDR-TBFQ+) or second-line injectable (MDR-TBSLI+) is suboptimal. The availability of BDQ and DLM in the country provided an opportunity to evaluate the effectiveness of an all-oral shorter regimen for patients with pulmonary MDR-TBFQ+/SLI+.
METHODS
The BEAT-India (Building Evidence to Advance Treatment of TB) study, a prospective open-label, single-group cohort study, enrolled participants at 5 sites in India between April 2019 and January 2021: Indian Council of Medical Research (ICMR)–National Institute for Research in Tuberculosis and its satellite sites at the Government Hospital of Thoracic Medicine and the Government Thiruvoteeswarar Hospital of Thoracic Medicine, Chennai; Rajan Babu Institute of Pulmonary Medicine and Tuberculosis, in Delhi; National Institute of Tuberculosis and Respiratory Disease in New Delhi; the Group of Tuberculosis Hospital in Mumbai; and BJ Medical College Hospital in Ahmedabad. The study was initiated after obtaining approval from the institutional ethics committee of all the participating institutes and informed written consent from all study participants. The study was registered with the Clinical Trials Registry of India: (CTRI/2019/01/017310) and monitored by an Independent Data Safety Monitoring Committee consisting of a pharmacologist, pulmonologist, statistician, trialists, and cardiologist who met periodically as per the protocol.
Adults with pulmonary MDR-TBFQ+ or/and MDR-TBSLI+ with 2 positive sputum smears or at least 1 culture-positive with negative sputum smears collected within 6 weeks before the screening date were included in the study. Patients with Karnofsky scores of 50 or less, hepatitis B or C infection with abnormal liver function tests (LFTs; ie, aminotransferases >2.5 times above normal), human immunodeficiency virus (HIV) infection, grade 2 or above peripheral neuropathy (according to Division of AIDS [DAIDS] Table for Grading the Severity of Adult and Pediatric Adverse Events) [15], pre-existing cardiac disease, and pregnant or breast feeding were excluded from the study.
The designated study staff explained the risks (including the cardiotoxicity profile of study drugs and frequent clinic visits) and benefits (shorter duration of treatment and noninjectable regimen) to all eligible participants. Pretreatment evaluation was done as defined in the protocol (Supplementary Table 1). The critical concentrations for drug susceptibiity test (DST) of BDQ, LZD, and CFZ were 1 µg/mL and for DLM was 0.06 µg/mL, as recommended by WHO [16]. Eligible participants received BDQ 400 mg once daily for 2 weeks, followed by 200 mg 3 times weekly for 22 weeks; DLM 100 mg twice daily; LZD 600 mg once daily; and CFZ 100 mg or 200 mg based on their body weight for 24 weeks. In case of an LZD-induced grade 3 adverse event (AE), LZD dose could be reduced to 300 mg as predefined in the protocol.
Sputum, chest X-ray (CXR), and blood investigations were done during the treatment and posttreatment follow-up period as predefined in the protocol (Supplementary Table 1). Chest X-ray was evaluated for opacities and cavities in lung zones [17]. Participants were withdrawn from the study if any study drug resistance with culture positivity was found after week 8 of treatment. DAIDS criteria were used to assess the regimen’s safety and tolerability. Drug-related toxicity was treated promptly [15]. Fridericia-corrected QT intervals on electrocardiography (ECG) [QTc(f)] was graded based on Common Terminology Criteria for Adverse Events (CTCAE) [18]. At week 24, the decision to stop treatment was based on 16th-week mycobacterial growth indicator tube (MGIT) sputum culture: if the culture was negative, treatment was stopped; if not, treatment was extended for 12 weeks. Missed doses were compensated over 15 days to achieve 80% coverage of drug intake. Participants with a favorable outcome were followed until week 48 to rule out TB recurrence.
Treatment outcome was declared favorable if 2 consecutive sputum cultures taken at least 4 weeks apart were negative, with clinical (defined as the absence of signs and symptoms of TB, weight gain from pretreatment level) and radiological improvement (measured as decrease in the distribution of opacity, cavity, nodes from baseline, and absence of any new lesion on CXR) at the end of treatment. Patients with negative sputum cultures at the end of treatment but with no radiological or clinical improvement were not considered to have had a favorable outcome. Unfavorable outcomes included bacteriological failure (failure to attain culture conversion by week 28 and treatment extended to 36 weeks) or clinical failure (worsening of clinical symptoms or radiological features during treatment or adverse drug reactions warranting permanent treatment change). Failure to maintain culture negativity (2 positive consecutive sputum cultures taken at least 4 weeks apart) after a favorable treatment outcome was considered bacteriological recurrence.
Statistical Analysis
With an assumption that the new combination of the BEAT-India study regimen would achieve a 50% cure rate against the 30% cure under programmatic management of DR-TB, the estimated sample size was 127, with a population risk difference of within 10% of the actual value with 95% confidence. With a 20% loss due to death and 10% loss due to study withdrawal, the total sample size required was 165 participants. IBM SPSS software, version 25.0 (IBM Corporation), was used for all analyses. A modified intent-to-treat analysis (mITT) was done, excluding patients with sputum culture negative at inclusion and/or FQ sensitive and/or resistant to any study drugs. Standard time-to-event analysis using the Cox proportional hazards model estimated time to culture conversion. Age, gender, weight, diabetic status, initial sputum smear grade, baseline hemoglobin, number of zones involved, and presence of cavity on CXR were significant covariates influencing time to culture conversion. Unadjusted Cox and Accelerated Failure Time (AFT) models were used to estimate the effect of covariates on the hazard rate.
RESULTS
Patient Characteristics
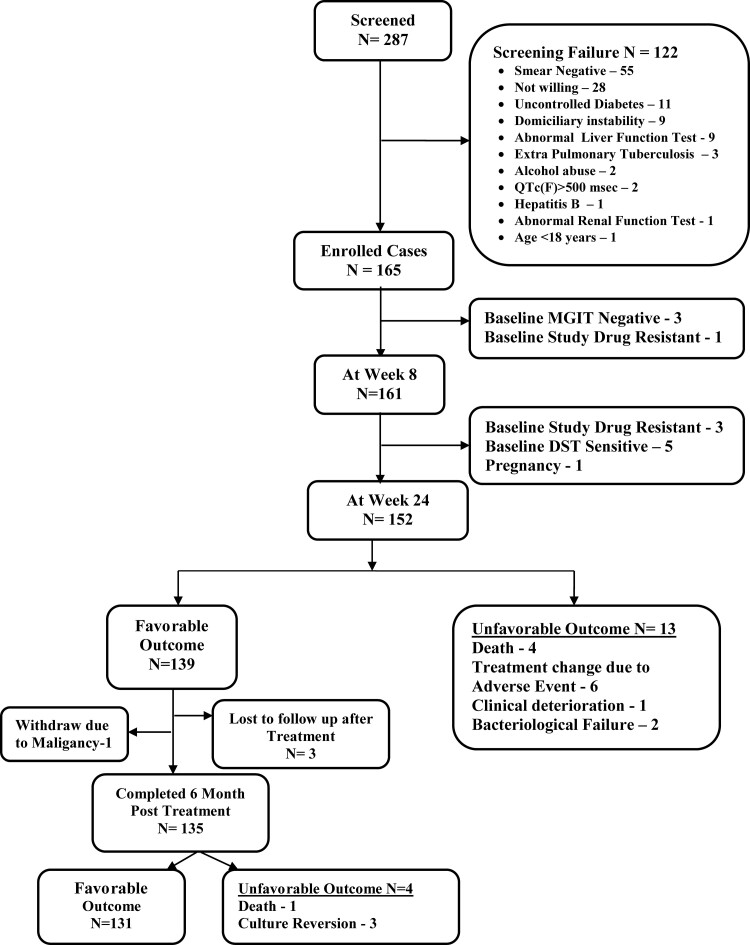
A total of 122 potential participants were excluded for various reasons (Figure 1), and 165 eligible patients were enrolled in the study. Table 1 shows the demographic and clinical characteristics of study participants. The majority of patients had pulmonary MDR-TBFQ+ with extensive disease involving both lungs. Patients were further classified based on WHO body mass index (BMI) cutoffs [19] and the extent of lung zone involvement [18]. All patients had previously received TB medications, the most common of which were FQ, pyrazinamide, isoniazid, ethambutol, and SLI. Baseline MGIT DST showed resistance in 4 patients: BDQ, LZD, and CFZ in 1 patient; BDQ and DLM in 1 patient; LZD and CFZ in 1 patient; and LZD alone in 1 patient.
Figure 1.
CONSORT checklist of participants in BEAT-India study. Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; DST, drug susceptibility test; MGIT, mycobacterial growth indicator tube.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants
| Characteristics | Values (N = 165) |
|---|---|
| Median age (range), years | 27.0 (18.0–56.0) |
| Median weight (range), kg | 46.0 (30.0–94.5) |
| Median BMI (range), kg/m2 | 17.3 (13.1–33.1) |
| Male gender | 92 (55.8%) |
| Presence of diabetes mellitus | 19 (11.5%) |
| Current smoker | 22 (13.3%) |
| Alcohol use | 24 (14.5%) |
| Categories of BMI | |
| <16.5 kg/m2 | 41 (24.8%) |
| 16.5–18.5 kg/m2 | 59 (35.8%) |
| >18.5 kg/m2 | 65 (39.4%) |
| Chest X-ray involvement | |
| Unilateral | 67 (40.6%) |
| Bilateral | 98 (59.4%) |
| Zones involved in chest X-ray | |
| ≤3 Zones | 108 (65.5%) |
| > 3 Zones | 57 (34.5%) |
| Cavity on chest X-ray | |
| Yes | 84 (50.9%) |
| No | 81 (49.1%) |
| Drug susceptibility pattern | |
| FQ resistant | 158 (95.8%) |
| FQ sensitive and SLI resistant | 7 (4.2%) |
| Sputum smear at baseline | |
| Negative | 8 (4.8%) |
| Scanty (1–29 AFB/30 fields) | 14 (8.5%) |
| 1+ | 49 (29.7%) |
| 2+ | 52 (31.5%) |
| 3+ | 42 (25.5%) |
| MGIT culture at baseline | |
| Positive | 162 (98.2%) |
| Negative | 3 (1.8%)a |
Data are presented an n (%) unless otherwise indicated. 1+: (30-299 SFB/1 length)/2+:10-100 AFB/1 field/3+: >100 AFB/1 field. Abbreviations: BMI, body mass index; FQ, fluoroquinolone; MGIT, mycobacterial growth indicator tube; SLI, second-line resistance.
Enrolled based on earlier sputum culture.
Effectiveness Analysis
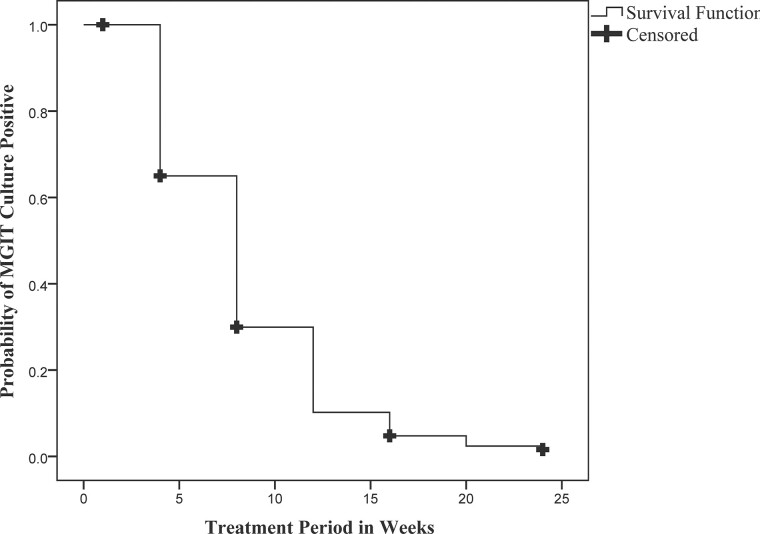
At the end of 24 weeks of treatment, 7 patients were sputum culture–positive at the16th week; hence, their treatment was extended for 36 weeks. Twelve patients were excluded due to baseline study drug resistance (4), culture-negative (3), or FQ and SLI sensitivity (5). Therefore, at the end of treatment, by mITT, 139 of 153 patients (91%; 95% CI: 85–95%) had favorable outcomes (Table 2). By the eighth week of treatment, 118 of 157 patients (75%) had culture converted. The average time for smear conversion was 9.5 weeks (95% CI: 8.4–10.5 weeks), and for culture, conversion was 8.5 weeks (95% CI: 7.8–9.2 weeks) (Figure 2). By the Cox proportional hazards model, all of the covariates listed contributed to time to culture conversion (hazard ratio >1.0), but none were statistically significant. By the AFT model, Mycobacterium tuberculosis (M.tb) bacilli load (based on initial sputum smear grading) and disease extent (based on the number of lung zones involved on CXR) were predictors of the time to culture conversion. Patients with baseline sputum smear grading of negative/1+ converted 1.45 times faster than those with higher sputum positivity of 2+/3+ (P = .025). Similarly, the involvement of fewer than 3 lung zones resulted in 1.43 times faster culture conversion than when more than 3 lung zones involved (P = .039). At the end of treatment, all patients had gained at least 3.0 kg of body weight and had significant radiological improvement. Fourteen patients (9%) had unfavorable outcomes—4 deaths during treatment, 7 treatment changes (6 due to adverse drug reactions [ADRs]), 1 due to clinical deterioration and 2 bacteriological failures (Figure 1). One patient withdrew from the study after week 10 due to pregnancy. Of the 6 patients with treatment change due to ADRs, 2 had QTc(f) prolongation at week 8, 2 showed grade 3 liver enzyme elevation at week 6, 1 showed grade 2 pancreatic enzymes increase at week 12, and 1 patient had blurring of vision. For QTc prolongation and vision disturbances, the BDQ-DLM regimen was permanently stopped, while for enzyme elevation, treatment was temporarily withheld. In case of no improvement and if the sputum remained positive, the BDQ-DLM regimen was permanently withdrawn, and patients started on an alternative regimen.
Table 2.
Showing the Effectiveness of the BEAT Study Regimen Among Patients Enrolled in the BEAT-India Study
| Outcome | FQ Resistant | FQ Sensitive, SLI Resistant | Overall |
|---|---|---|---|
| Total no. of patients | 158a | 7 | 165 |
| Modified intent-to-treat population | |||
| Withdrawal based on drug sensitivity profile/culture negativity | 10 | 2 | 12 |
| No. of patients | 148 | 5 | 153 |
| Favorable outcome | |||
| No. of patients | 134 | 5 | 139 |
| Percentage of patients (95% CI) | 91 (85–95) | 100 (47–100) | 91 (85–95) |
| Unfavorable outcome | 14b | 0 | 14b |
Abbreviations: CI, confidence interval; FQ, fluoroquinolone; SLI, second-line resistance.
Sixteen patients had both FQ and SLI resistance.
One withdrawal from treatment during week 10 due to pregnancy.
Figure 2.
Kaplan-Meier plot showing the time to sputum culture conversion among the participants on the study regimen. Abbreviation: MGIT, mycobacterial growth indicator tube.
Safety Analysis
Thirty-three serious AEs were reported in 26 patients, including death and hospitalizations due to various causes such as grade 3 or 4 anemia, peripheral neuropathy, liver enzyme elevation, etc (Table 3). Three participants developed severe breathlessness during the treatment period due to coronavirus disease 2019 (COVID-19) infection, necessitating hospitalization. Four patients died during treatment: 1 due to massive hemoptysis during the third week, 1 due to septicemia during the fourth week, and 2 due to respiratory failure between the 15thand 19th weeks of treatment. While on treatment, various drug-related AEs were noticed among participants, and most of them were identified early and managed successfully (Table 4). Of the 139 patients with favorable outcomes, 94 (68%) had completed the entire regimen without interruption or dose reduction, whereas 45 (32%) had either an interruption or dose reduction due to AEs. Six of the 13 patients with unfavorable outcomes had their treatment interrupted.
Table 3.
Serious Adverse Events Among BEAT Study Participants During the Treatment Period
| No. | Events | Number of Participants With Any Events (n = 33) | Median Time to First Appearance | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Resolved | Deaths | ||||||||
| 1 | Breathlessness for evaluationa | 10 | 11th week | 1 | 4 | 1 | 4 | 8 | 2 |
| 2 | Fever for evaluation | 1 | 8th week | … | 1 | … | … | 1 | … |
| 3 | Gastritis and vomiting | 3 | 19th week | … | … | 3 | … | 3 | … |
| 4 | Haemoptysis | 4 | 10th week | 2 | 1 | … | 1 | 3 | 1 |
| 5 | Pneumothorax | 2 | 8th week | … | 2 | … | … | 2 | … |
| 6 | Acute exacerbation of asthma | 1 | 10th week | … | … | … | 1 | 1 | … |
| 7 | Anemia | 6 | 13th week | … | … | 1 | 5 | 6 | … |
| 8 | Peripheral neuropathyb | 1 | 21st week | … | … | … | 1 | … | … |
| 9 | Pancreatic enzyme elevation | 1 | 24th week | … | 1 | … | … | 1 | … |
| 10 | Liver enzyme elevation | 2 | 6th week | … | 2 | … | … | 2 | … |
| 11 | Septic shock | 1 | 4th week | … | … | … | 1 | … | 1 |
| 12 | Urinary tract infection | 1 | 21st week | … | … | … | … | 1 | … |
Thirty-three events in 26 patients: 5 patients had 2 events/1 patients had 3 events.
Abbreviations: COVID-19, coronavirus disease 2019; LZD, linezolid.
Three patients initially admitted for breathlessness but later they tested positive for COVID-19.
One patient developed peripheral neuropathy during the 18th week so the LZD dose was reduced and later withheld until hospital admission.
Table 4.
Drug-related adverse events among BEAT study participants while on treatment
| S.No | System | Count | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Resolved | Unresolved | |
|---|---|---|---|---|---|---|---|---|---|
| Under follow-up | Lost to follow-upa | ||||||||
| 1 | Skin | 97 (59%) | … | … | … | … | 81 | … | … |
| ȃ1.Hyperpigmentation | 91 (55%) | 84 | 7 | 0 | 0 | 75 | 10 | 6 (UFO: 3 / BLWD: 3) |
|
| ȃ2. Acne | 6 (4%) | 5 | 1 | 0 | 0 | 6 | … | … | |
| 2 | Hematology | 85 (52%) | … | … | … | … | 81 | … | … |
| ȃ1. Anemia | 78 (47%) | 43 | 18 | 14 | 3 | 74 | … | 4 (UFO: 2 / BLWD: 2) |
|
| ȃ2. Thrombocytopenia | 7 (4%) | 5 | 2 | 0 | 0 | 7 | … | … | |
| 3 | Enzyme elevation | 77 (47%) | … | … | … | … | 73 | … | … |
| ȃ1. SGOT or SGPT | 38 (23%) | 36 | 2 | 0 | 0 | 37 | 1 | … | |
| ȃ2. Both SGOT and SGPT | 37 (22%) | 31 | 4 | 2 | 0 | 34 | … | 3 (UFO: 3) |
|
| ȃ3. Serum bilirubin | 2 (1%) | 2 | 0 | 0 | 0 | 2 | … | … | |
| 4 | Peripheral neuropathy | 69 (42%) | 58 | 11 | 0 | 0 | 52 | 10 | 7 (UFO: 5 / BLWD: 2) |
| 5 | Pancreatic enzyme elevation | 24 (15%) | … | … | … | … | 18 | … | … |
| ȃ1. Serum amylase | 10 (6%) | 9 | 1 | 0 | 0 | 6 | 4 | … | |
| ȃ2. Serum lipase | 6 (4%) | 5 | 1 | 0 | 0 | 5 | 1 | … | |
| ȃ3. Amylase and lipase | 8 (5%) | 4 | 3 | 1 | 0 | 7 | … | 1 (BLWD: 1) |
|
| 6 | CVS | 43 (11%) | … | … | … | … | 41 | … | … |
| ȃ1. QTc prolongation | 40 (10%) | 12 | 1 | 27 | 0 | 38 | … | 2 (UFO: 2) |
|
| ȃ2. Chest pain | 3 (1%) | 2 | 1 | 0 | 0 | 3 | … | … | |
Abbreviations: BLWD, baseline study withdrawal done during study conduct due to culture negativity or study drug resistance; CVS, cardiovascular system; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; UFO, unfavorable study outcome so participant was withdrawn from further follow-up.
Classification of grading based on Common Terminology Criteria for Adverse Events (CTCAE) grading.
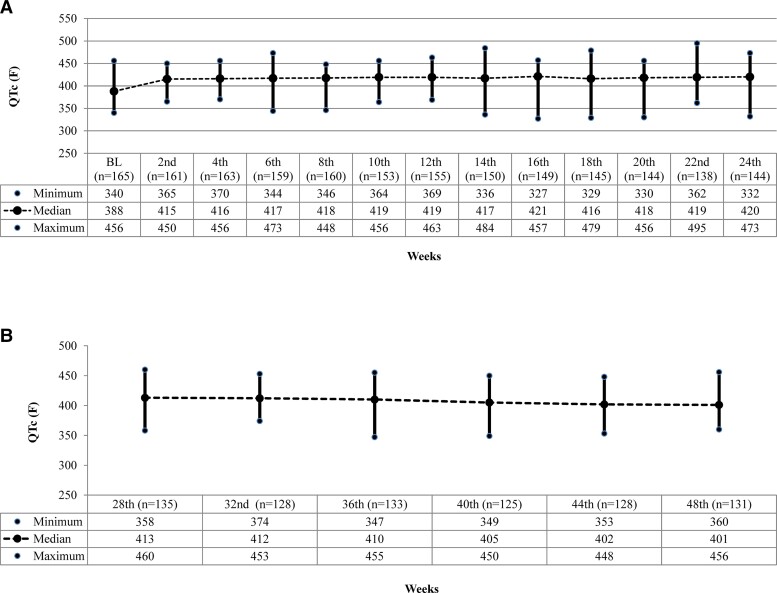
Skin AEs were the most common, with 97 patients developing hyperpigmentation or acneiform eruptions during treatment. Anemia of varying severity developed in 78 patients, the majority after 8 weeks of treatment. Peripheral neuropathy was reported in 69 patients, mostly between 16 and 24 weeks (Table 4). Linezolid was temporarily withheld for 7–14 days in patients with grade 3 or 4 severities, and symptoms were managed appropriately. The first LZD dose reduction or interruption for neuropathy occurred during the seventh week and for anemia during ninth week of treatment. Linezolid was reintroduced at a lower dose of 300 mg in 45 patients after symptoms subsided. By the end of treatment, 40 of those given a lower dose of LZD achieved cure. Treatment was discontinued in 2 patients after 14 weeks—1 due to clinical deterioration and the other due to optic neuritis; 3 patients were withdrawn from the study due to DST. There was no QTc(f) prolongation of more than 500 ms. Four patients with QTc(f) prolongation had their treatment interrupted for 7–14 days. One patient died and 2 had persistent ECG changes after removal of the suspected drug; hence, they were started on an alternate regimen. The remaining 37 patients completed their treatment and were declared cured. Figure 3 depicts the median QTc(f) during treatment and 6 months thereafter.
Figure 3.
A, Median QTc(f) on ECG among BEAT-India study participants during the treatment phase. B, Median QTc(f) on ECG in BEAT-India study participants during the post-treatment follow-up period. Abbreviation: ECG, electrocardiography.
During post-treatment follow-up of the 139 patients with favorable outcome, 1 patient was withdrawn from the study due to malignancy. At the 48th week, 131 patients (94%) had sustained treatment success, 3 (2%) had recurrent TB, 3 were lost to follow-up, and 1 died at week 28 due to respiratory distress. Resolution of drug-related AEs was noticed in most patients: fading of hyperpigmentation (84%), reversal of peripheral neuropathy (75%), drug-induced anemia resolved in all patients after treatment completion.
DISCUSSION
Our findings show that a fully oral, short-course regimen of BDQ and DLM with other drugs gives a favorable outcome of 91% in patients with MDR-TBFQ+/SLI+ and 69% in those with both FQ and SLI resistance. The median time to culture conversion was 8 weeks. The favorable outcome of this regimen is greater than that seen in a South African cohort study where a BDQ-DLM combination was used along with moxifloxacin, CFZ, or pyrazinamide [11] but similar to that seen in the EndTB observational study [20]. The majority of patients in our cohort had extensive bilateral disease and high bacterial load in sputum, yet we achieved higher culture conversion at the end of short treatment regimen. This is likely because the combination of BDQ and DLM with repurposed drugs had a good response to treatment. Our results are also comparable to that of the NixTB trial, which used a combination of BDQ, LZD, and pretomanid (Pa) with a favorable outcome of 90% [21] and also the TB-PRACTECAL study with BDQ, Pa, LZD, moxifloxacin, which showed a favorable outcome of 89% [22].
Treating DR-TB poses several hurdles to physicians and national TB programs due to the restricted number of drugs available and limited experience with newer regimens. This study generated additional evidence on efficacy of 1 such combination that can be tried in the presence of contraindications or nonavailability of Pa. In situations where BPaLM (Bedaquiline, Pretomanid, Linezolid, Moxifloxacin) regimen cannot be used, the BEAT study regimen can be offered. However, the effect of these newer drugs/regimens on different lineages of M.tb is yet to be studied. In countries where LZD is used to treat MDR-TB but baseline DST is unavailable, physicians may be hesitant to use a combination of 2 new drugs with only LZD. The BEAT regimen, which has shown a satisfactory treatment outcome, can be used in such instances.
The majority of the AEs were easily identifiable, reversible, and related to LZD (anemia) and CFZ (hyperpigmentation) rather than BDQ. This will reduce physicians’ apprehension about using BDQ-containing regimens in nonhospital settings due to cardiotoxicity concerns. Combining BDQ and DLM as salvage therapy has shown promising results, with minimal effect on QTc interval and no arrhythmias [14, 23, 24]. The DELIBERATE trial demonstrated good culture conversion and only a minor impact on QTc interval with combined use of BDQ and DLM [25]. Our cohort’s average increase in QTc from baseline was 30–45 ms, slightly higher than in South African cohorts [25, 26]; this could be due to the concomitant use of 3 potentially QT-influencing drugs. However, our cohort, which included larger number of patients using this drug combination than previously reported [11, 13], did not encounter any patient with QTc(f) of more than 500 ms. This reassuringly supports the concurrent use of BDQ and DLM in patients with MDRFQ+.
Hyperpigmentation secondary to CFZ use observed in our cohort was similar to the report from a Brazilian cohort [27]. Discontinuing the offending agent at the end of treatment resulted in the reversal of hyperpigmentation in most of our patients. Our cohort’s proportion of patients with anemia was comparable to other studies [11, 21]. Still, peripheral neuropathy was higher than in the South African study, probably due to factors such as alcohol abuse, malnutrition, or diabetes mellitus. Unlike the NixTB trial, our patients received 600 mg of LZD daily, with 34 patients requiring further dose reduction to 300 mg/day due to AEs, and all of them had a favorable outcome. A retrospective study from Georgia [28] found similar results with LZD dose reduction. These findings suggest that a 600-mg/day dose may be sufficient when combined with BDQ-DLM. However, the effect of a lower dose of LZD on TB recurrence requires further research.
The major strength of our study is close monitoring of patients with a detailed schedule of investigations. As the study regimen had 3 potentially QTc-prolonging drugs, ECG was taken frequently both during and post-treatment, unlike as suggested by various guidelines [29, 30]. In our experience, performing an ECG before treatment initiation and then at 8, 16, 24, 32, or 40 weeks after treatment initiation is sufficient. Similarly, we suggest monthly monitoring of clinical symptoms, complete blood count, and liver function test (LFT) for early detection of AEs. Serum potassium, calcium, and magnesium should be done whenever QT prolongation is noticed and corrected promptly. Other investigations like serum albumin, amylase, and lipase can be done as and when indicated.
Lack of a concurrent comparator group could be a limitation in interpreting the study results. It will not be easy to distinguish the regimen effect from the overall effect, limiting the study’s validity. When this study was conducted in the country, the national program’s standard of care was a more extended regimen with an injectable, with poor success and high mortality rates [31]. As a result, having a concurrent comparator arm was considered unethical. At the same time, because it was conducted in the field, this study has greater generalizability and can be replicated in other resource-constrained settings. High success rates highlight the need for a better, shorter, and more patient-friendly regimen to reduce clinic visits and hospital expenses for patients, families, and the healthcare system. The combination of BDQ with DLM has shown promising results in patients with MDR-TB who had previously been exposed to FQ. This combination can also be considered in earlier stages of the disease and in countries with access to Pa and DLM.
Conclusions
This study demonstrates that MDR-TBFQ+ can be successfully treated with an entirely oral, short-course regimen of BDQ and DLM in combination with repurposed drugs. Also, this combination makes it feasible to identify and correct the AEs early in field settings. Based on emerging evidence, short-course regimens should be made the standard of care and initiated early in managing DR-TB.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Chandrasekaran Padmapriyadarsini, ICMR–National Institute for Research in Tuberculosis, Chennai, India.
Vikram Vohra, National Institute for Tuberculosis and Respiratory Diseases, New Delhi, India.
Anuj Bhatnagar, Rajan Babu Institute of Pulmonary Medicine and Tuberculosis, Delhi, India.
Rajesh Solanki, B. J. Medical College and Hospital, Ahmedabad, India.
Rathinam Sridhar, Government Hospital of Thoracic Medicine, Chennai, India.
Lalitkumar Anande, Grand TB Hospital, Mumbai, India.
M Muthuvijaylakshmi, ICMR–National Institute for Research in Tuberculosis, Chennai, India.
Meera Bhatia Rana, National Institute for Tuberculosis and Respiratory Diseases, New Delhi, India.
Bharathi Jeyadeepa, ICMR–National Institute for Research in Tuberculosis, Chennai, India.
Gaurav Taneja, Rajan Babu Institute of Pulmonary Medicine and Tuberculosis, Delhi, India.
S Balaji, ICMR–National Institute for Research in Tuberculosis, Chennai, India.
Prashant Shah, B. J. Medical College and Hospital, Ahmedabad, India.
N Saravanan, ICMR–National Institute for Research in Tuberculosis, Chennai, India.
Vijay Chavan, Grand TB Hospital, Mumbai, India.
Hemanth Kumar, ICMR–National Institute for Research in Tuberculosis, Chennai, India.
Chinnayin Ponnuraja, ICMR–National Institute for Research in Tuberculosis, Chennai, India.
Viktoriya Livchits, US Agency for International Development, Washington D.C., USA.
Monica Bahl, Clinical Development Service Agency, New Delhi, India.
Umesh Alavadi, US Agency for International Development, Washington D.C., USA.
K S Sachdeva, Central TB Division, Ministry of Health and Family Welfare, New Delhi, India.
Soumya Swaminathan, Indian Council of Medical Research, New Delhi, India; World Health Organization, Geneva, Switzerland.
for the BEAT India Team:
C Padmapriyadarsini, B Jeyadeepa, Lakshana, Nabila Akbar, Edwin Arulraj, Karthikeyan, Muthukumar, Tamizharasan, S Balaji, S Shivakumar, M Muthuvijayalakshmi, Gayathri, C Ponnuraja, Hemanth Kumar, N Saravanan, R Sridhar, R Kumar, Ramesh, Vikram Vohra, Meera Bhatia Rana, Neeta Singla, V P Myneedu, Ananiya Lawrence, Dipti Kushwaha, Deepak Kheraliya Shivam, Rohit Sarin, Anuj K Bhatnagar, Gaurav Taneja, Alok Rawat, M Haniff, Rahul, Padma Rai, Savita Saini, Krishan Kumar Mathur, Rajesh N Solanki, Pranav G Patel, Vaidehi Prajapati, Bhavesh Parmar, Kajal Wadkar, Prashant L Shah, Snehal Parmar, Palak Vyas, Krupa Mistri, Lalitkumar Anade, Vijay Chavan, Namrata Kaur Bhui, Pranita Tipre, Daksha Shah, Surendra K Patwa, Anis Nhavakar, Audrey Brito, Kiran Keny, Vijaykumar Karanjkar, Kuntal Pal, Komal Godam, Madri Huje, Sanjana Ghadge, Madhuri Udmalle, Vivek Vijay Posture, Jaipal Bansode, Monica Bhal, Ranjan, Divya Pillai, Supriya Semwal, Shirali Labroo Viktoriya Livchits, Umesh Alavadi, Reuben Swamikan, Dorothy Nanzala Nasubo, Mallik Parmar, Suvanad Sahu, YaDiul Mukadi, and Soumya Swaminathan
Notes
Acknowledgments. The authors are grateful to all the participants of the BEAT study. They extend their sincere gratitude and special thanks to all clinical, laboratory, and administrative staff of the participating institutes, without whose untiring help this project would not have been completed on time, especially during the coronavirus disesase 2019 (COVID-19) pandemic and nationwide lockdown. A special mention to the STOP TB Partnership team and the US Agency for International Development (USAID) team for their support and guidance during the study.
Disclaimer. The contents of this study manuscript are the authors’ sole responsibility and do not necessarily reflect the views of the USAID or the US government.
Financial support. This work was supported by the American people through the USAID and the Indian Council of Medical Research. B. J. reports support for this manuscript for medical writing.
BEAT study team members. C. Padmapriyadarsini, B. Jeyadeepa, Lakshana, Nabila Akbar, Edwin Arulraj, Karthikeyan, Muthukumar, Tamizharasan, S. Balaji, S. Shivakumar, M. Muthuvijayalakshmi, Gayathri, C. Ponnuraja, Hemanth Kumar, N. Saravanan, R. Sridhar, R. Kumar, Ramesh, Vikram Vohra, Meera Bhatia Rana, Neeta Singla, V. P. Myneedu, Ananiya Lawrence, Dipti Kushwaha, Deepak Kheraliya Shivam, Rohit Sarin, Anuj K. Bhatnagar, Gaurav Taneja, Alok Rawat, M.Haniff, Rahul, Padma Rai, Savita Saini, Krishan Kumar Mathur, Rajesh N. Solanki, Pranav G. Patel, Vaidehi Prajapati, Bhavesh Parmar, Kajal Wadkar, Prashant L. Shah, Snehal Parmar, Palak Vyas, Krupa Mistri, Lalitkumar Anade, Vijay Chavan, Namrata Kaur Bhui, Pranita Tipre, Daksha Shah, Surendra K. Patwa, Anis Nhavakar, Audrey Brito, Kiran Keny, Vijaykumar Karanjkar, Kuntal Pal, Komal Godam, Madri Huje, Sanjana Ghadge, Madhuri Udmalle, Vivek Vijay Posture, Jaipal Bansode, Monica Bhal, Ranjan, Divya Pillai, Supriya Semwal, Shirali Labroo Viktoriya Livchits, Umesh Alavadi, Reuben Swamikan, Dorothy Nanzala Nasubo, Mallik Parmar, Suvanad Sahu, YaDiul Mukadi, and Soumya Swaminathan.
References
- 1.World Health Organization. Global tuberculosis report 2021 . Geneva, Switzerland: World Health Organization, 2021. Available from:https://www.who.int/publications/i/item/9789240037021. Accessed 18 September 2021.
- 2. Pym AS, Diacon AH, Tang SJ, et al. . Bedaquiline in the treatment of multidrug and extensively drug-resistant tuberculosis. Eur Respir J 2016; 47:564–74. doi: 10.1183/13993003.00724-2015. [DOI] [PubMed] [Google Scholar]
- 3. Schnippel K, Ndjeka N, Maartens G, et al. . Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med 2018; 6:699–706. doi: 10.1016/S2213-2600(18)30235-2. [DOI] [PubMed] [Google Scholar]
- 4. Seung KJ, Khan P, Franke MF, et al. . Culture conversion at 6 months in patients receiving delamanid-containing regimens for the treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2020; 71:415–8. doi: 10.1093/cid/ciz1084. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva, Switzerland: World Health Organization, 2019. [PubMed] [Google Scholar]
- 6. Sotgiu G, Pontali E, Migliori GB. Linezolid to treat MDR-/XDR-tuberculosis; available evidence and future scenarios. Eur Respir J 2015; 45:25–9. doi: 10.1183/09031936.00145014. [DOI] [PubMed] [Google Scholar]
- 7. Singh B, Cocker D, Ryan Het al. . Linezolid for drug-resistant pulmonary tuberculosis. Cochrane Database Syst Rev 2019;3:CD012836. doi: 10.1002/14651858.CD012836.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Falagas ME, Vardakas KZ, et al. . Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid-containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. J Thorac Dis 2015; 7:603. doi: 10.3978/j.issn.2072-1439.2015.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nugraha RV, Yunivita V, Santoso Pet al. . Clofazimine as a treatment for multidrug-resistant tuberculosis: a review. Sci. Pharm 2021; 89:19. doi: 10.3390/scipharm89020019. [DOI] [Google Scholar]
- 10. Ammerman NC, Swanson RV, Bautista EM, et al. . Impact of clofazimine dosing on treatment shortening the first-line regimen in a mouse model of tuberculosis. Antimicrob Agents Chemother 2018; 62:1–18. doi: 10.1128/AAC.00636-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olayanju O, Esmail A, Limberis J, et al. . A regimen containing bedaquiline and delamanid compared to bedaquiline in patients with drug-resistant tuberculosis. Eur Respir J 2020; 55:1901181. doi: 10.1183/13993003.01181-2019. [DOI] [PubMed] [Google Scholar]
- 12. Maryandyshev A, Pontali E, Tiberi S, et al. . Bedaquiline and delamanid combination treatment of 5 patients with pulmonary extensively drug-resistant tuberculosis. Emerg Infect Dis 2017; 23:1718–21. doi: 10.3201/eid2310.170834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferlazzo G, Mohr E, Laxmeshwar C, et al. . Early safety and efficacy of the combination of bedaquiline and delamanid for the treatment of patients with drug-resistant tuberculosis in Armenia, India, and South Africa: a retrospective cohort study. Lancet Infect Dis 2018; 18:536–44. doi: 10.1016/S1473-3099(18)30100-2. [DOI] [PubMed] [Google Scholar]
- 14. Guglielmetti L, Barkane L, Le Dû D, et al. . Safety and efficacy of exposure to bedaquiline-delamanid in multidrug-resistant tuberculosis: a case series from France and Latvia. Eur Respir J 2018; 51:1702550. doi: 10.1183/13993003.02550-2017. [DOI] [PubMed] [Google Scholar]
- 15. DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, corrected version 2.1. Available at:https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. Accessed 21 September 2021.
- 16.World Health Organization. Technical report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. Geneva, Switzerland: World Health Organization, 2018 (WHO/CDS/TB/2018.5). Available at:https://apps.who.int/iris/handle/10665/260470. Accessed 22 September 2021.
- 17. Chest X-ray: survival guide. Available at:https://www.elsevier.com/books/the-chest-x-ray-a-survival-guide/unknown/978-0-7020-3046-8. Accessed 7 February 2021.
- 18.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 published: 27 November 2017. Available at:https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf. Accessed 12 May 2022.
- 19. Weir CB, Jan A. BMI classification percentile and cut-off points. Available at:https://www.ncbi.nlm.nih.gov/books/NBK541070/. Accessed 2 October 2021. [PubMed]
- 20. Franke MF, Khan P, Hewison C, et al. . Culture conversion in patients treated with bedaquiline and/or delamanid. Am J Respir Care Med 2021; 203:929. doi: 10.1164/rccm.202001-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Conradie F, Diacon AH, Ngubane N, et al. . Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med 2020; 382:893–902. doi: 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. TB PRACTECAL: MSF clinical trial finds short, effective, and safe drug-resistant tuberculosis treatment. Available at:https://msf.org.uk/article/tb-practecal-msf-clinical-trial-finds-short-effective-and-safe-drug-resistant-tuberculosis. Accessed 15 May 2022.
- 23. Lachatre M, Rioux C, Du DL, et al. . Bedaquiline plus delamanid for XDR tuberculosis. Lancet Infect Dis 2016; 16:294. doi: 10.1016/S1473-3099(16)00047-5. [DOI] [PubMed] [Google Scholar]
- 24. Kim CT, Kim TO, Shin HJ, et al. . Bedaquiline, and delamanid for the treatment of multidrug-resistant tuberculosis: a multicenter cohort study in Korea. Eur Respir J 2018; 51:1702467. doi: 10.1183/13993003.02467-2017. [DOI] [PubMed] [Google Scholar]
- 25. Dooley KE, Rosenkranz SL, Conradie F, et al. . QT Effects of bedaquiline, delamanid, or both in patients with rifampicin-resistant tuberculosis: a phase 2, open-label, randomized, controlled trial. Lancet Infect Dis 2021; 21:975–83. doi: 10.1016/S1473-3099(20)30770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Isralls S, Baisley K, Ngam Eet al. . QT interval prolongation in people treated with bedaquiline for drug-resistant tuberculosis under programmatic conditions: a retrospective cohort study. Open Forum Infect Dis 2021; 8:ofab413. doi: 10.1093/ofid/ofab413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dalcolmo M, Gayoso R, Sotgiu G, et al. . Effectiveness and safety of clofazimine in multidrug-resistant tuberculosis: a nationwide report from Brazil. Eur Respir J 2017; 49:1602445. doi: 10.1183/13993003.02445-2016. [DOI] [PubMed] [Google Scholar]
- 28. Mikiashvili L, Kipiani M, Schechter MC, et al. . Linezolid use for drug-resistant tuberculosis in Georgia: a retrospective cohort study. Int J Tuberc Lung Dis 2020; 24:436–43. Doi: 10.5588/ijtld.19.0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guidelines for programmatic management of drug-resistant tuberculosis in India. March 2021. Available at: https://tbcindia.gov.in/showfile.php. Accessed 12 May 2022.
- 30. WHO operational handbook on tuberculosis. Module 4: treatment—drug-resistant tuberculosis treatment. Geneva, Switzerland: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
- 31. India TB report 2021. Available at:https://tbcindia.gov.in/showfile.php?lid=3587. Accessed 22 September 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.