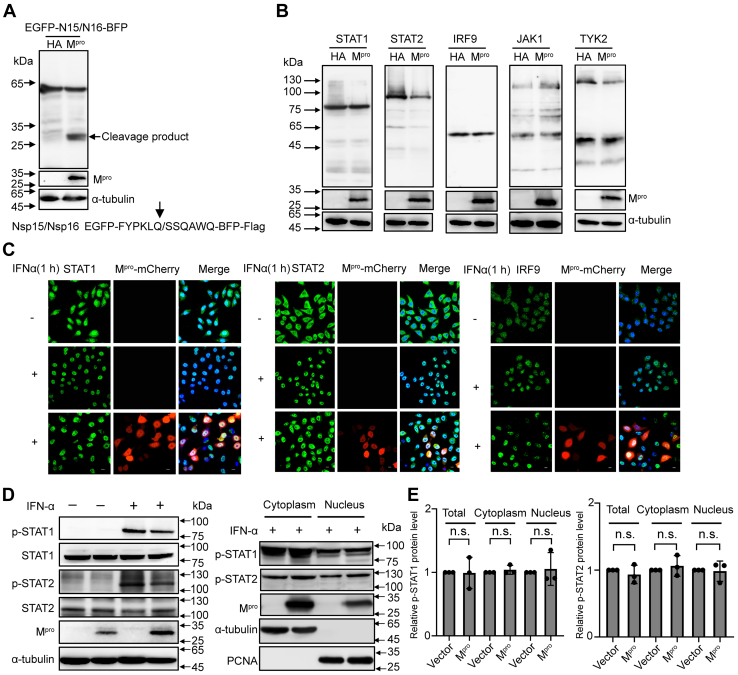
Figure 2.
Mproshows no interaction with IFN signaling pathway transducers.A, HEK-293T cells were transfected with a C-terminal flag-tagged EGFP-N15/N16-BFP plasmid along with SARS-CoV-2 Mpro or empty vector. Cells were lysed at 24 h after transfection and analyzed by Western blotting. B, HEK-293T cells were cotransfected with a C-terminal flag-tagged STAT1, STAT2, IRF9, JAK1, or TYK2 expression plasmid combined either with SARS-CoV-2 Mpro or empty vector. Whole-cell extracts were lysed 30 h posttransfection and analyzed by Western blotting. C, HeLa cells were transfected with pCDNA3.1-Mpro-mCherry visual construct, and 24 h posttransfection, cells were treated with or without IFNα (1000 U/ml) for 1 h and possessed for indirect immunofluorescence to detect the STAT1, STAT2, and IRF9. Scale bar, 10 μm. D, HEK-293T cells were transfected with SARS-CoV-2 Mpro or empty vector. After 24 h of expressing, cells were cultured with or without IFNα (1000 U/ml) for 1 h. Whole-cell extracts or the nuclear and cytoplasmic fractions were analyzed by Western blotting with specific antibodies for the detection of tyrosine phospho-STAT1 and STAT2 or total STAT1 and STAT2. E, density analysis represents the relative protein levels of phospho-STAT1 or phospho-STAT2 that was normalized to the protein levels of α-tubulin or PCNA. The value of control group was set to 1. The presented results represent the means and standard deviations of data from three independent experiments. Statistical significance was calculated using unpaired, two-tailed Student’s t test. Protein band intensities were quantitated by Image Lab software. IFN, interferon; IRF9, interferon regulatory factor 9; Mpro, main protease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; STAT, signal transducer and activator of transcription.