Figure 5. Overview of the dynamic among RTMs and disease-associated mo-macs in tissues and leveraging it for translational science.
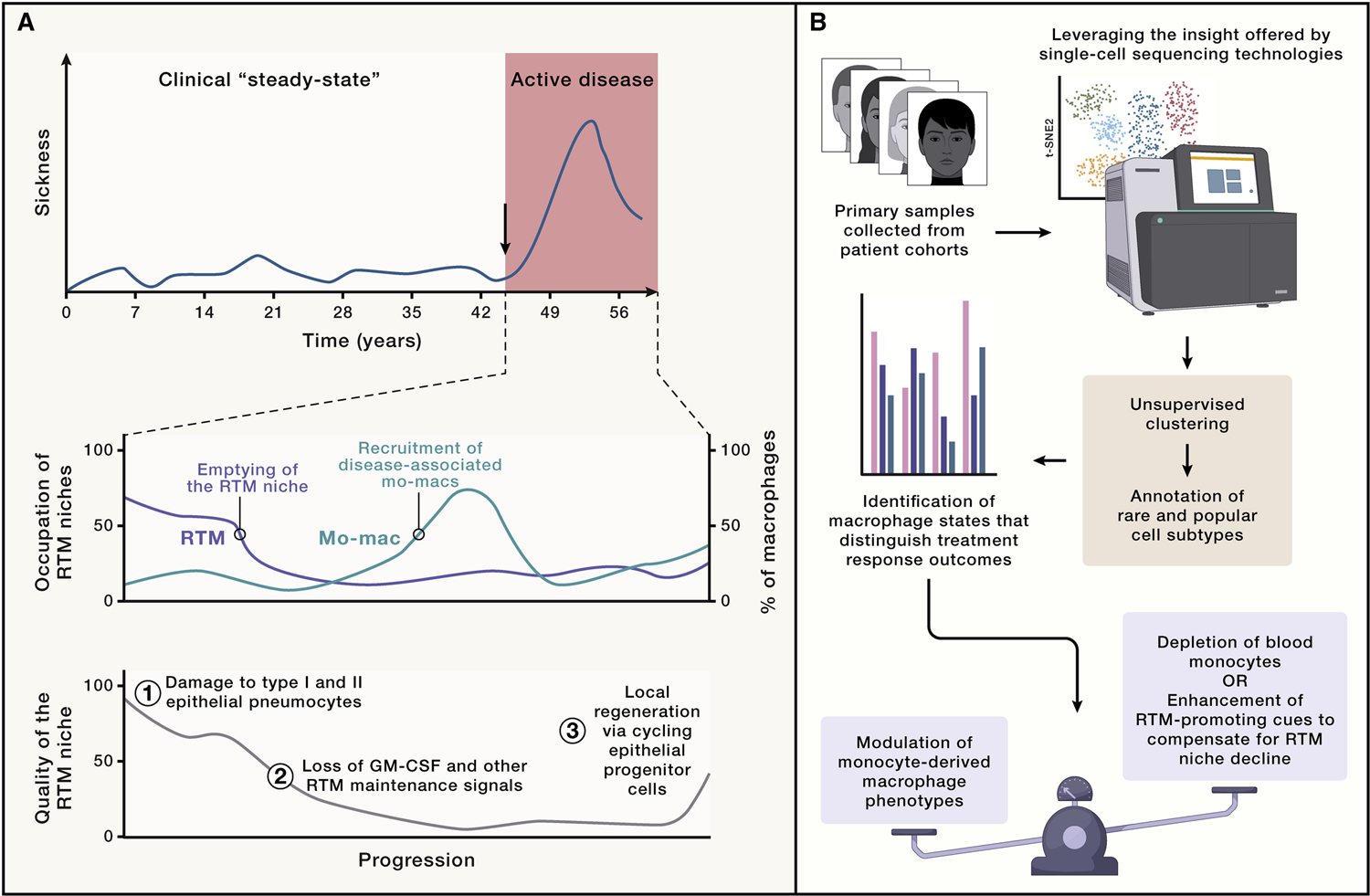
(A) Delineating the occupational dynamic of tissues by tissue-resident macrophages (RTMs) (blue) and monocyte-derived macrophages (mo-macs) generated during disease (red) will not only elucidate how their functional differences drive disease in humans but also at what points during disease would response to certain therapies be most appreciated. (B) In order to do so, insight must be driven by precise, scientific questions with translational potential. The advent and continued use of single-cell technologies enable the scientific community to do that, though other methods of highly rigorous profiling efforts could do the same. Broadly, considering the uncertainty of whether mo-macs at disease resolution help revive the local pool of RTMs and the fact that cell states among mo-macs are wide-ranging and diverse, modulating their phenotype—as opposed to trying to deplete them entirely or outcompete them by attempting to forcibly expand surviving RTMs during disease—will be the more practical outlook on new therapeutic designs.
