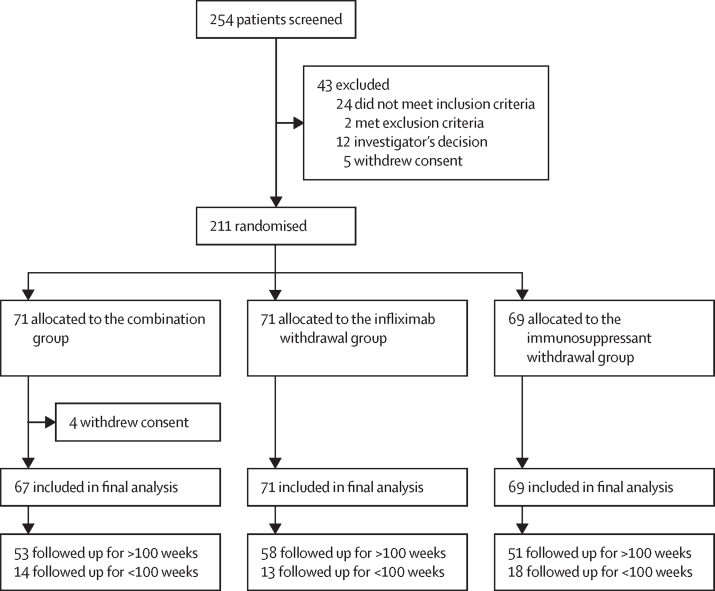
Figure 2.
Trial profile
Reasons for a follow-up duration shorter than 2 years (<100 weeks, per protocol) in the trial were: Combination group: treatment failure (n=6), patient's decision (n=5), physician's decision (n=2), and last follow-up visit performed too early (n=1); infliximab withdrawal group: treatment failure (n=5), physician decision (n=1), patient's decision (n=4), and last follow-up visit performed too early (n=3); immunosuppressant withdrawal group: treatment failure (n=11), physician's decision (n=1), patient's decision (n=1), last follow-up visit performed too early (n=1), lost to follow-up (n=2), and pregnancy (n=2).