Figure 2. Common mechanisms of action by which ncRNAs regulate cellular function.
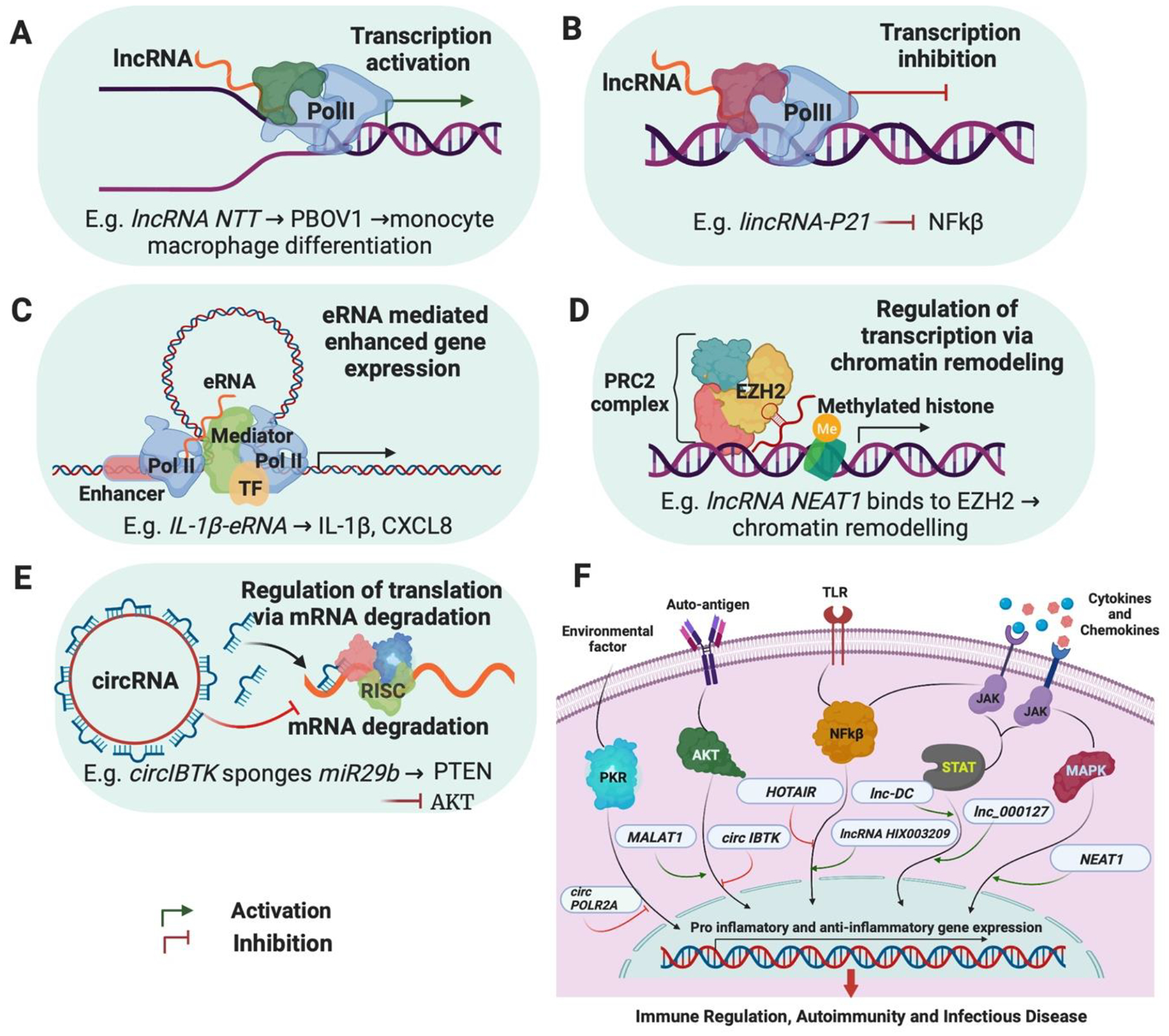
Shown in A-E are examples of how ncRNAs regulate cellular function, with exemplar genes included. A, activation of gene transcription. A common mechanism is physical interactions that facilitate recruitment of the transcriptional machinery to the promotors of target genes to enhance transcription. B, inhibition of gene transcription. Here, lncRNAs can act as decoys and bind to transcription factors to alter their recruitment or that of polymerase II to the promoter, resulting in transcriptional suppression. C, enhancement of gene transcription. eRNAs transcribed from enhancer elements commonly function to stabilize enhancer-promoter interactions via DNA looping and recruit transcription factors and polymerase II. D, chromatin remodeling. ncRNAs, for example lncRNAs, can interact with chromatin remodeling complex proteins, such as EZH2 and PRC2 (promoting polycomb repressor complexes), modulating histone modification and thereby gene expression. E, competing endogenous RNAs. Some ncRNAs, such as lncRNAs and circRNAs can act as sponges for miRs, thereby modulating the degradation of mRNAs that are the cognate targets of those miRs. F, many of these mechanisms can converge to regulate biological pathways in cells. Shown are just some of the cellular processes implicated in the pathogenesis of autoimmune diseases and how ncRNAs could impact pro- and anti-inflammatory gene expression programs in those pathways. TLR, Toll Like Receptor; PKR, Protein Kinase RNA; AKT, Protein Kinase B; NFκB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; STAT, Signal Transducer and Activator of Transcription; MAPK, Mitogen-Activated Protein Kinase; JAK, Janus Kinase.
