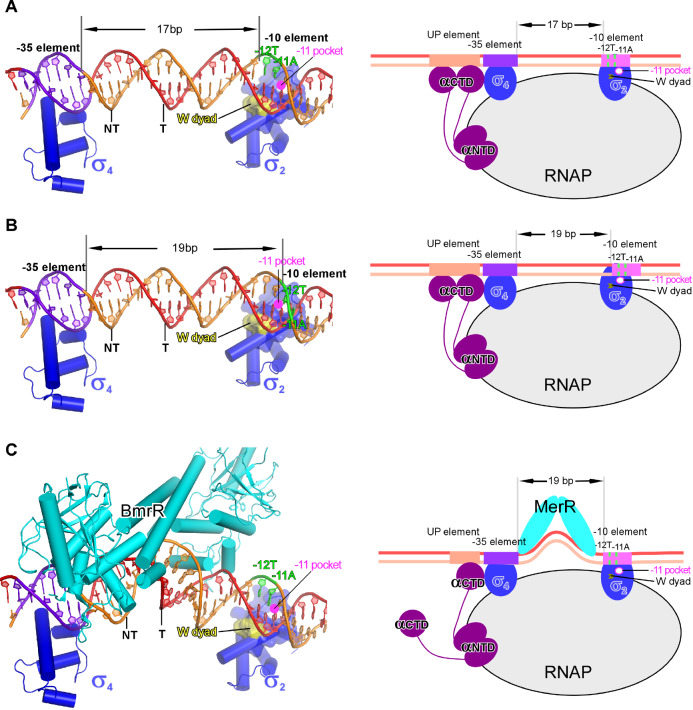
Figure6 .
The DNA-distortion mechanism of transcription activation by MerR-family TFs(A) The –35 and –10 elements are properly aligned on the protein surface of σ4 and σ2. The –11A of the nontemplate strand DNA is close to the –11 pocket. The panel figure is prepared from a B. subtilis RPc model comprising a promoter DNA with 17-bp –35/–10 spacer. (B) The –11A of the nontemplate strand DNA is rotated away from the –11 pocket in the B. subtilis RPc model comprising a promoter DNA with 19-bp –35/–10 spacer. (C) The BmrR-induced central kink realigns the −35 and −10 elements to a proper space and phase for simultaneous engagement by σ4 and σ2 according to the structure model of a BmrR-RPc complex. Yellow surfaces (left) and arrows (right) show the tryptophan dyad; pink circles show the –11 pocket.