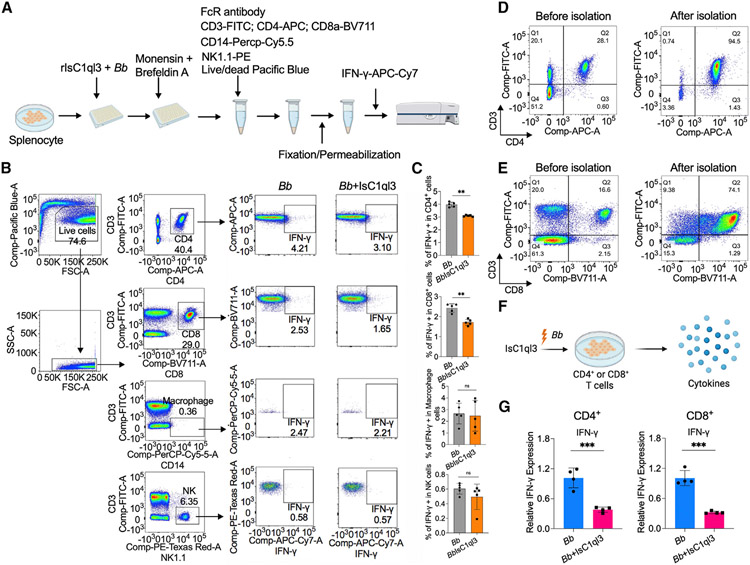
Figure 5. Tick salivary protein IsC1ql3 inhibits B. burgdorferi-induced IFN-γ production by mainly targeting CD4+ and CD8+ T cells.
(A) Work flow to detect IFN-γ production in CD4+, CD8+ T cells, macrophages, and NK cells after incubating with B. burgdorferi (1 × 106 cells/mL) alone or mixture with 1 μg/mL rIsC1ql3 for 6 h by flow cytometry.
(B) Flow cytometry and gating strategy to quantify IFN-γ production in CD4+, CD8+ T cells, macrophages, and NK cells.
(C) Percentage of CD4+, CD8+ T cells, macrophages, and NK cells expressing IFN-γ. IsC1ql3 significantly inhibits IFN-γ production in CD4+ and CD8+ cells upon B. burgdorferi infection. Each dot represents one biological replicate. Data are represented as mean ± SD. Statistical significance was assessed using a non-parametric Mann-Whitney test (**p < 0.01).
(D) Isolation of CD4+ T cells from splenocytes. The purity of the isolated CD4+ T cells was determined by flow cytometry.
(E) Isolation of CD8+ T cells from splenocytes. The purity of the isolated CD8+ T cells was determined by flow cytometry.
(F) The purified CD4+ or CD8+ T cells were incubated with spirochetes (1 × 106 cells/mL) alone or as a mixture with 1 μg/mL rIsC1ql3 for 6 h. The IFN-γ gene expression was then evaluated by qPCR.
(G) IsC1ql3 significantly inhibits IFN-γ gene expression in CD4+ or CD8+ T cells upon B. burgdorferi infection. Each dot represents one biological replicate. Data are represented as mean ± SD. Statistical significance was assessed using a non-parametric Mann-Whitney test (*p < 0.05).