Fig. 1.
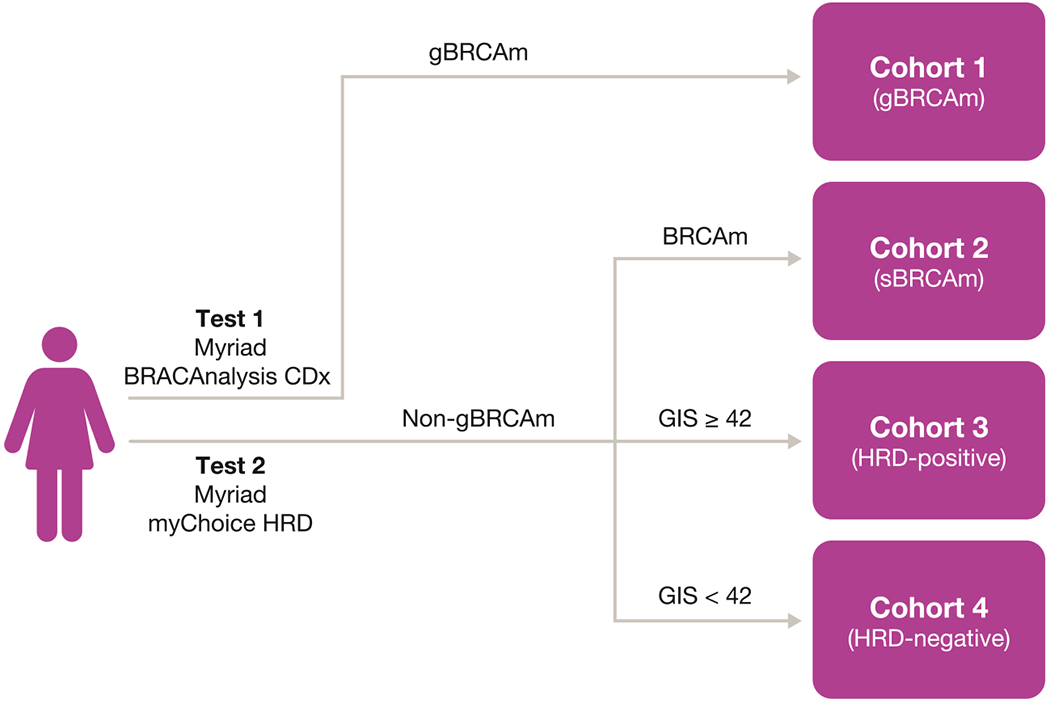
Patient assignment to cohorts in the LIGHT study.
The Myriad myChoice® HRD assay was FDA-approved as a companion diagnostic assay for olaparib in October 2019 and renamed myChoice CDx®. BRCAm, BRCA1 and/or BRCA2 mutation; FDA, US Food and Drug Administration; gBRCAm, germline BRCAm; GIS, genomic instability score; HRD, homologous recombination deficiency; LIGHT, oLaparib In Germline-, HRD-, and Tumor-mutated versus wild-type ovarian cancer; sBRCAm, somatic BRCA mutation.
