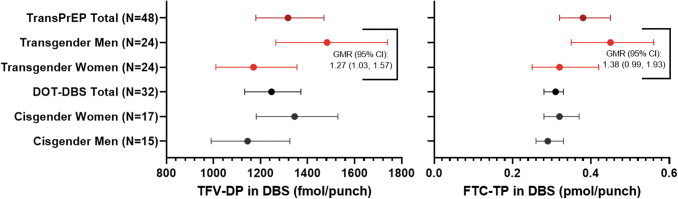
FIG. 3.
Week 4 concentrations of TFV-DP and FTC-TP in DBS. All concentrations are shown as geometric mean (95% CI). Concentrations of TFV-DP and FTC-TP are shown for the overall study population, TW, and TM in red. The DOT-DBS study was a randomized crossover study in which participants received directly observed TDF/FTC for 12 weeks.13 They were assigned to two DOT treatment regimens of 33%, 67%, or 100% daily dosing, separated by a 12-week washout. Since all TransPrEP participants were instructed to take TDF/FTC daily, only those participants randomized to 100% daily dosing were included in this summary. DBS concentrations from the DOT-DBS study following 4 weeks of 100% dosing (i.e., week 4 or 28 for those randomized to 100% dosing for the first or second dosing regimen, respectively) are shown in black for the overall study population, cisgender women, and cisgender men. TDF, tenofovir disoporoxil fumarate.