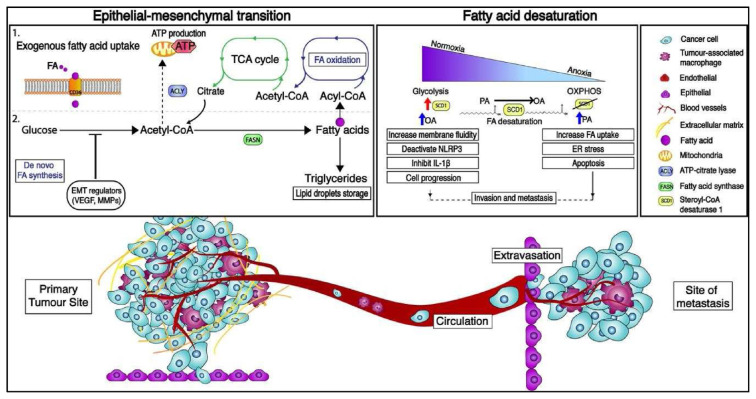
Fig. 1.
The metabolic characteristics of the epithelial-to-mesenchymal transition (EMT) as well as an overview of the metastatic cascade. Several components of the molecular pathways that drive EMT have a major impact on cell metabolism and vice versa, leading in metabolic rewiring within the glycolysis, tricarboxylic acid (TCA) cycle, and fatty acid production pathways. Exogenous absorption and de novo lipogenesis are two methods by which cancer cells obtain fatty acids (FAs). CD36, a specialised transporter, facilitates exogenous FA uptake from the environment. FAs can then be stored as lipid droplets and integrated into triglycerides for energy storage, as well as used to produce acetyl-coA via FA-oxidation. Cancer cells also use glucose as a carbon source for de novo lipogenesis in order to synthesise citrate. The enzymatic activity of ATP-citrate lyase (ACLY), fatty acid synthase (FASN), and acyl-coA carboxylase produce FAs from citrate (ACC). Furthermore, depending on the oxygen tension, EMT-committed cancer cells may rely on an aerobic glycolytic metabolism or shift toward oxidative phosphorylation (OXPHOS). The suppression of stearoyl-CoA desaturase 1 (SCD1) in cancer cells produces endoplasmic reticulum (ER) stress and apoptosis, demonstrating that decreasing oxygen tension causes changes in metabolism via fatty acid desaturation. VEGF stands for vascular endothelial growth factor, MMP stands for matrix metalloproteinases, and NLRP3 stands for NLR family pyrin domain containing 3.