Figure 1.
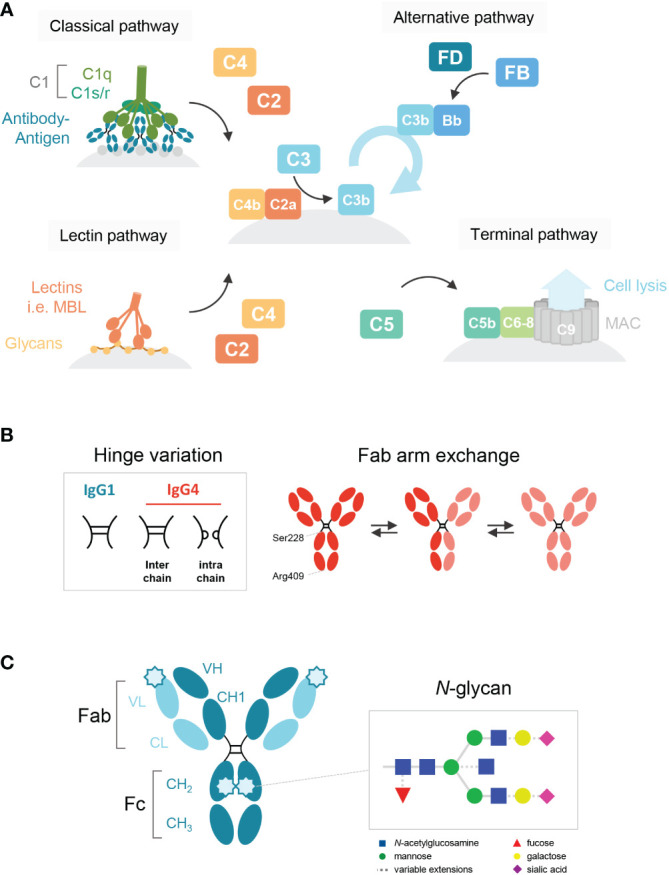
Schematic overview of the human IgG antibody structure and antibody-mediated complement activation. (A) Generalized overview of the complement system, which is activated through three pathways: the classical, lectin and alternative pathway. The classical pathway is activated by binding of C1q to antigen-bound IgM and IgG, which then forms the C1 complex with its proteases C1r/s. The lectin pathway is activated by the binding of lectins, such as mannose-binding lectin (MBL), to carbohydrates on cell surfaces. C3b deposition induced by these pathways or spontaneous hydrolysis of C3 can activate the alternative pathway, mediated by Factor B and D, which results in an amplification loop of C3b deposition. C3b can opsonize the target cell for phagocytosis and can lead to the assembly of the membrane attack complex with factors C5b to C9 through the terminal pathway and subsequent cell lysis. (B) A structural representation of the hinge variation and half molecule exchange of IgG4 via switch of inter-chain to intra-chain disulfide bonds at amino acid positions 228 and 409. (C) General structure of an IgG monomer and schematic representation of N-linked glycan composition of human IgG Abs. The glycans are attached to asparagine (N) at position 297 in the CH2 domain and have a biantennary heptasaccharide core (solid line) and variable extensions (dash line), such as fucose, galactose and/or sialic acid. Novel glycosylation sites can be introduced within the Fab region of matured antibodies.
