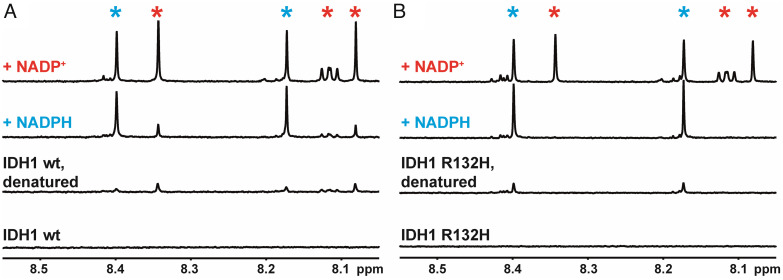
Fig. 1.
1H NMR data showing the release of copurified enzyme-bound NADP(H) from wild-type IDH1 (A) and IDH1 R132H (B) upon thermal denaturation (see SI Appendix, Fig. S3 for the equivalent experiment with FNR). Reading vertically from the lower spectra i) folded IDH1 protein—NADP(H) is initially enzyme-bound, hence not observed; ii) denaturation releases NADP(H) into solution. (A) Wild-type IDH1 copurifies with an approximately 2:1 mixture of NADP+ (red asterisk) and NADPH (blue asterisk). (B) IDH1 R132H apparently copurifies exclusively with NADPH.